Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals
Abstract
:1. Introduction
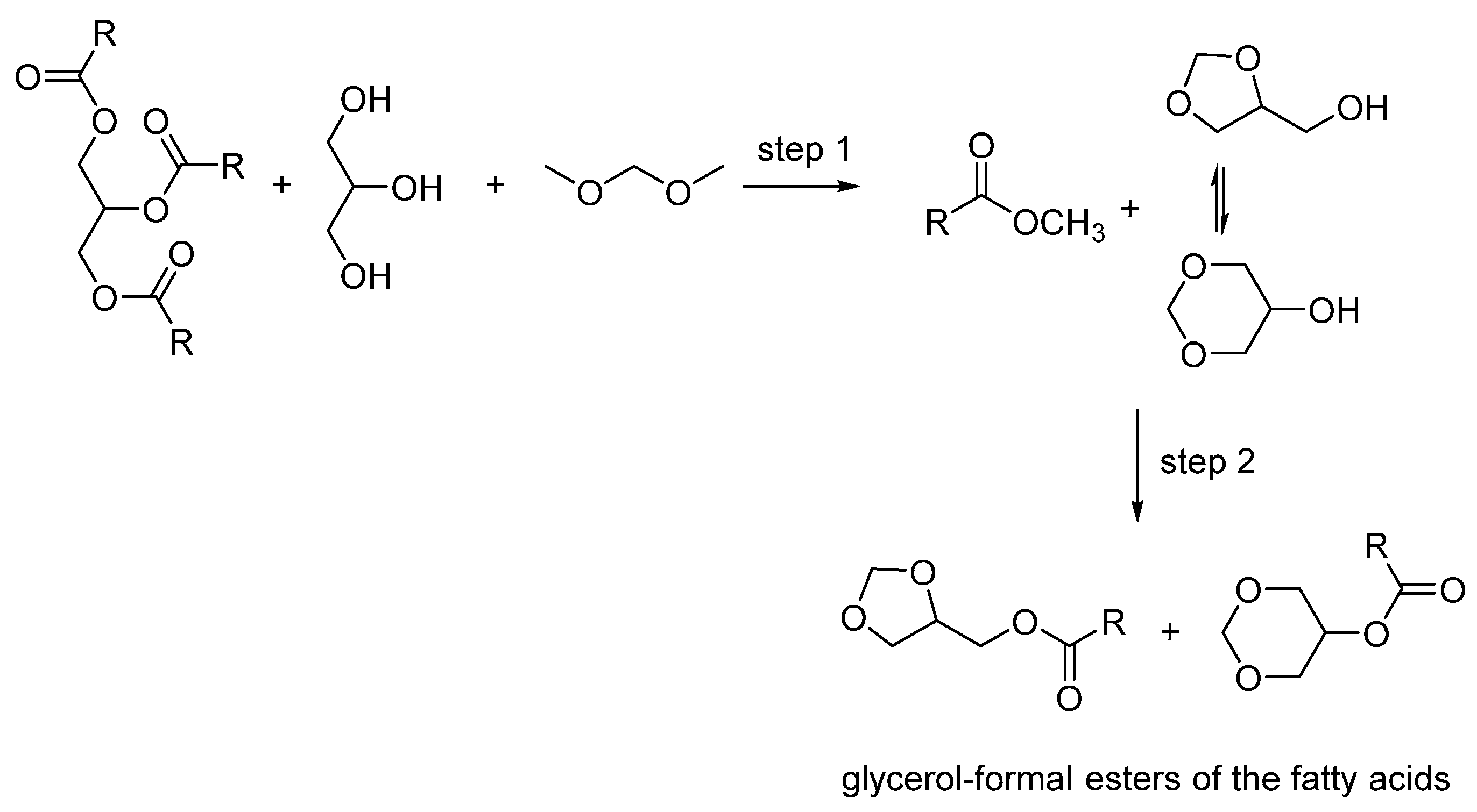
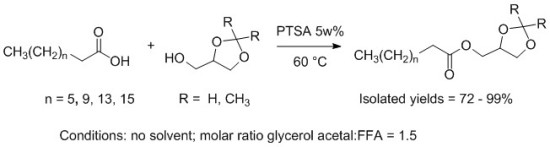
2. Results and Discussion

2.1 Synthesis of the Fatty Acid Solketal Esters (FASEs)

| FFA | mol × 10−3 | Solketal b (mol × 10−3) | PTSA c (g) | Product | Isolated Yield (%) | Purity (% GC) | Melting Point (°C) |
|---|---|---|---|---|---|---|---|
| C8:0 | 13.80 | 20.7 | 0.1 | 1 | 92 | > 99 | liquid |
| C12:0 | 9.98 | 15.0 | 0.1 | 2 | 80 | 97 | 40–48 |
| C16:0 | 7.79 | 11.7 | 0.1 | 3 | > 99 | 92 | 55 |
| C18:0 | 7.03 | 10.5 | 0.1 | 4 | 87 | >99 | 54 |
2.2 Synthesis of the Fatty Acid Glycerol Formal Esters (FAGEs)

| FFA | mol × 10−3 | GlyF b (mol × 10−3) | PTSAc (g) | Product | Isolated Yield (%) | Purity (% GC) | Melting Point (°C) |
|---|---|---|---|---|---|---|---|
| C8:0 | 13.80 | 20.7 | 0.1 | 5 | 72 | 86 | liquid |
| C12:0 | 9.98 | 15.0 | 0.1 | 6 | 91 | 76 | 35–38 |
| C16:0 | 7.79 | 11.7 | 0.1 | 7 | 97 | 97 | 51–54 |
| C18:0 | 7.03 | 10.5 | 0.1 | 8 | 97 | > 99 | 66–70 |
3. Experimental Section
3.1 Materials and Methods
3.2 Characterization
3.3 General Procedure for Synthesis of Fatty Acid Solketal Esters (FASEs)
3.4 General Procedure for Synthesis of Fatty Acid Glycerol Formal esters (FAGE)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and challenges for biodiesel fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Liaquat, A.M.; Kalam, M.A.; Masjuki, H.H.; Jayed, M.H. Potential emissions reduction in road transport sector using biofuel in developing countries. Atmos. Environ. 2010, 44, 3869–3877. [Google Scholar] [CrossRef]
- Quispea, C.A.G.; Coronadoc, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energ. Rev. 2013, 27, 475–493. [Google Scholar]
- Ciriminna, R.; Della Pina, C.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, K.; Zhang, X.; Liu, S.; Wang, M.; Deng, L.; Wang, F.; Tan, T. Production of fumaric acid from biodiesel derived crude glycerol by Rhizopus arrhizus. Bioresour. Technol. 2014, 163, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lapuerta, M.; Rodríguez-Fernandez, J.; Estevez, C.; Bayarri, N. Properties of fatty acid glycerol formal ester (FAGE) for use as a component in blends for diesel engines. Biomass Bioenerg. 2015, 76, 130–140. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Z.; Wang, W.; Lu, X. Microbial recycling of glycerol to biodiesel. Bioresour. Technol. 2013, 150. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Garcia, E.; Laca, M.; Perez, E.; Garrido, A.; Peinado, J. New class of acetal derived from glycerin as biodiesel fuel component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Selva, M.; Benedet, V.; Fabris, M. Selective Catalytic Etherification of Glycerol Formal and Solketal with Dialkyl Carbonates and K2CO3. Green Chem. 2012, 14, 188–200. [Google Scholar] [CrossRef]
- Selva, M.; Guidi, S.; Noè, M. Upgrading of glycerol acetals by thermal catalystfree transesterification of dialkyl carbonates under continuous-flow conditions. Green Chem. 2015, 17, 1008–1023. [Google Scholar] [CrossRef]
- Haider, M.H.; Dummer, N.F.; Knight, D.W.; Jenkins, R.L.; Howard, M.; Moulijn, J.; Taylor, S.H.; Hutchings, G.J. Efficient green methanol synthesis from glycerol. Nat. Chem. 2015, 7, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, C.; di Blasio, G.; Lazzaro, M.; Cannilla, C.; Bonura, G.; Frusteri, F.; Asdrubali, F.; Baldinelli, G.; Presciutti, A.; Fantozzi, F.; et al. Technologies for energetic exploitation of biodiesel chain derived glycerol: Oxy-fuels production by catalytic conversion. Appl. Energ. 2013, 102, 63–71. [Google Scholar] [CrossRef]
- Gras, J.L.; Nouguier, R.; Mchich, M. Transacetalisation de triols a partir du dimethoxymethane. Selectivite et applications synthetiques. Tetrahedron Lett. 1987, 28, 6601–6604. [Google Scholar] [CrossRef]
- Da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- De Torres, M.; Jiménez-osés, G.; Mayoral, J.A.; Pires, E.; de los Santos, M. Glycerol ketals: Synthesis and profits in biodiesel blends. Fuel 2012, 94, 614–616. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Stepan, E.; Dragomir, R.E.; Radu, A.; Rosca, P. Synthesis and testing of glycerol ketals as components for diesel fuel. Fuel Process. Technol. 2013, 110, 214–217. [Google Scholar] [CrossRef]
- Song, J.; Zello, V.; Boehman, J. Energy Fuels. Performance and Emissions of a Compression Ignition Engine Fueled with Diesel/Oxygenate Blends for Various Fuel Delivery Advance Angles. Energy Fuels 2005, 19, 403–410. [Google Scholar]
- Boot, M.; Frijters, P.; Liijten, C.; Somers, B.; Baert, R.; Donkerbroek, A.; Klein-Douwel, R.J.H.; Dam, N. Cyclic Oxygenates: A New Class of Second-Generation Biofuels for Diesel Engines? Energy Fuels 2009, 23, 1808–1817. [Google Scholar]
- Soares, V.L.P.; Lachter, E.R.; Rodrigues, A., Jr.; Batista, L.N.; Nascimento, R.S.V. New Applications for Soybean Biodiesel Glycerol in Soybean—Applications and Technology, 1st ed.; Ng, T.-B., Ed.; InTech: Rijeka, Croatia, 2011; Volume 4, pp. 151–172, and references therein. [Google Scholar]
- Cablewsky, T.; Faux, A.F.; Strauss, C.R.J. Development and Application of a Continuous Microwave Reactor for Organic Synthesis. J. Org. Chem. 1994, 59, 3408–3412. [Google Scholar] [CrossRef]
- Sahai, P.; Vishwakarma, R.A.J. Phospholipase-A2-mediated stereoselective synthesis of (R)-1-O-alkylglycero-3-phosphate and alkyl-acyl analogues: Application for synthesis of radiolabelled biosynthetic precursors of cell surface glycoconjugates of Leishmania donovani. J. Chem. Soc. Perkin Trans. 1 1997, 12, 1845–1850. [Google Scholar] [CrossRef]
- Clarkson, J.S.; Walker, A.J.; Wood, M.A. Continuous Reactor Technology for Ketal Formation: An Improved Synthesis of Solketal. Org. Process Res. Dev. 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Delfort, B.; Durand, I.; Jaecker, A.; Lacome, T.; Montagne, X.; Paille, F. Diesel Fuel Compounds Containing Glycerol Acetals. U.S. Patent 2003163949, 4 September 2003. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Weisspapir, M. Topical Composition for Acne Treatment. U.S. Patent 2005255133, 17 November 2005. [Google Scholar]
- Moeller, H. A process for the Preparation of Salts of 1,3-Dioxolane-4-carboxylic Acids and Their Use. Patent DE3447783, 10 July 1986. [Google Scholar]
- Walsh Reed, H. Dioxolanes and Thio Analogs, Derivating Thereof and Lubricants and Fuels Containing Same. Patent WO8805071, 14 July 1988. [Google Scholar]
- Lee Dosuk, D. Delayed-Setting Calcium Phosphate Pastes. Patent WO2005117919, 15 December 2005. [Google Scholar]
- Fraatz, K.; Mertin, D.; Heep, I. Controlled Release System. U.S. Patent 2006034926, 16 February 2006. [Google Scholar]
- Vicente, G.; Melero, J.A.; Morale, G.; Paniagua, M.; Martin, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Jaecker-Voirol, A.; Durand, I.; Hillion, G.; Delfort, B.; Montagne, X. Glycerin for New Biodiesel Formulation. Oil Gas Sci. Technol. 2008, 63, 395–404. [Google Scholar] [CrossRef]
- Farmer, T.J.; Mascal, M. Platform Molecules in Introduction to Chemicals from Biomass, 2nd Ed.; Clark, J., Deswarte, F., Eds.; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Ozorio, L.P.; Pianzolli, R.; Mota, M.B.S.; Mota, C.J.A. Reactivity of Glycerol/Acetone Ketal (Solketal) and Glycerol/Formaldehyde Acetals toward Acid-Catalyzed Hydrolysis. J. Braz. Chem. Soc. 2012, 23, 931–937. [Google Scholar] [CrossRef]
- Goodby, J.W.; Watson, M.J.; Macenzie, G.; Kelly, S.M.; Bachir, S.; Bault, P.; Gode, P.; Goethals, G.; Martin, P.; Ronco, G.; Villa, P. The dependence of mesomorphic behaviour on the extent of hydrogen-bonding in sugar derived polyols. Liquid Cryst. 1998, 25, 139–147. [Google Scholar] [CrossRef]
- Milkereit, G.; Garamus, V.M.; Veermans, K.; Willumeit, R.; Vill, V. Synthesis and mesogenic properties of a Y-shaped glyco-glycero-lipid. Chem. Phys. Lipids 2004, 131, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perosa, A.; Moraschini, A.; Selva, M.; Noè, M. Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals. Molecules 2016, 21, 170. https://doi.org/10.3390/molecules21020170
Perosa A, Moraschini A, Selva M, Noè M. Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals. Molecules. 2016; 21(2):170. https://doi.org/10.3390/molecules21020170
Chicago/Turabian StylePerosa, Alvise, Andrea Moraschini, Maurizio Selva, and Marco Noè. 2016. "Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals" Molecules 21, no. 2: 170. https://doi.org/10.3390/molecules21020170
APA StylePerosa, A., Moraschini, A., Selva, M., & Noè, M. (2016). Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals. Molecules, 21(2), 170. https://doi.org/10.3390/molecules21020170