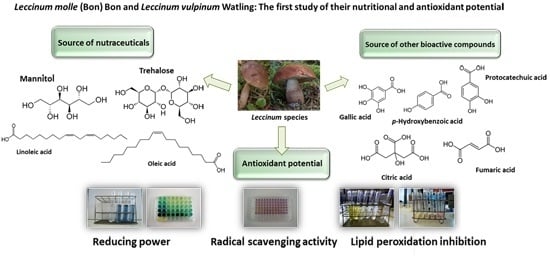
Leccinum molle (Bon) Bon and Leccinum vulpinum Watling: The First Study of Their Nutritional and Antioxidant Potential
Abstract
:1. Introduction
2. Results
2.1. Nutrient Composition
2.2. Non-Nutrient Composition
2.3. Antioxidant Potential
3. Discussion
3.1. Nutrient Composition
3.2. Non-Nutrient Composition
3.3. Antioxidant Potential
4. Experimental Section
4.1. Standards and Reagents
4.2. Mushroom Species and Sample Preparation
4.3. Nutrient Composition
4.3.1. Nutritional Value
4.3.2. Soluble Sugars
4.3.3. Fatty Acids
4.3.4. Tocopherols
4.4. Non-Nutrient Composition
4.4.1. Organic Acids
4.4.2. Phenolic Acids
4.5. Antioxidant Properties
4.5.1. Extraction Procedure
4.5.2. Antioxidant Activity Evaluation
- Reducing power
- Radical scavenging activity
- Lipid peroxidation inhibition
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kibby, G. A user-friendly key to the genus Leccinum in Great Britain. Field Mycol. 2000, 1, 20–29. [Google Scholar] [CrossRef]
- Iwański, M.; Rudawska, M. Ectomycorrhizal colonization of naturally regenerating Pinus sylvestris L. seedlings growing in different micro-habitats in boreal forest. Mycorrhiza 2007, 17, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Saxén, R.; Ilus, E. Transfer and behaviour of 137Cs in two Finnish lakes and their catchments. Sci. Total Environ. 2008, 394, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Vaaramaa, K.; Solatie, D.; Aro, L. Distribution of 210Pb and 210Po concentrations in wild berries and mushrooms in boreal forest ecosystems. Sci. Total Environ. 2009, 408, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Bastias, B.A.; Taylor, A.F.S.; Cairney, J.W.G. Identification of laccase-like genes in ectomycorrhizal basidiomycetes and transcriptional regulation by nitrogen in Piloderma byssinum. New Phytol. 2003, 157, 547–554. [Google Scholar] [CrossRef]
- Chen, D.M.; Taylor, A.F.S.; Burke, R.M.; Cairney, J.W.G. Identification of genes for lignin peroxidases and manganese peroxidases in ectomycorrhizal fungi. New Phytol. 2001, 152, 151–158. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Analytical methods applied to the chemical characterization and antioxidant properties of three wild edible mushroom species from Northeastern Portugal. Food Anal. Methods 2014, 7, 645–652. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Vaz, J.A.; Vasconcelos, M.H.; Martins, A. Compounds from wild mushrooms with antitumor potential. Anticancer Agents Med. Chem. 2010, 10, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.J.; Morel-Kopp, M.-C.; Chen, W.; Tofler, G.H.; Ward, C.M. Effects of Omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Semin. Thromb. Hemost. 2013, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. The Chemistry of Fungi; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Baati, T.; Horcajada, P.; Gref, R.; Couvreur, P.; Serre, C. Quantification of fumaric acid in liver, spleen and unrine by high-performance liquid chromatography coupled to photodiode-array detection. J. Pharm. Biomed. Anal. 2011, 56, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.Z.; Mao, L.; Ye, X.L.; Miao, J. Simultaneous determination of oxalic, fumaric, maleic and succinic acids in tartaric and malic acids for pharmaceutical use by ion-supression reversed-phase high performance liquid chromatography. J. Pharm. Biomed. Anal. 1999, 19, 621–625. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.W.; Caccetta, R.A.A.; Puddey, I.B.; Croft, K.D. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2000, 27, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carocho, M.; Ferreira, I.C.F.R. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. The Structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolics profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Mushroom voucher specimens are available from the authors.

| Nutrients | Leccinum molle (Bon) Bon | Leccinum vulpinum Watling | p-Value |
|---|---|---|---|
| Ash (g/100 g dw) | 5.70 ± 0.55 | 13.61 ± 0.86 | <0.01 |
| Proteins (g/100 g dw) | 13.07 ± 1.00 | 10.48 ± 0.50 | 0.0156 |
| Fat (g/100 g dw) | 2.80 ± 0.16 | 2.97 ± 0.13 | 0.1090 |
| Carbohydrates (g/100 g dw) | 78.43 ± 0.86 | 72.94 ± 0.51 | 0.0007 |
| Energy (kcal/100 g dw) | 391.18 ± 0.99 | 360.41 ± 2.89 | <0.001 |
| Fructose (g/100 g dw) | 3.06 ± 0.03 | 4.52 ± 0.14 | <0.0001 |
| Mannitol (g/100 g dw) | 11.32 ± 0.30 | 2.68 ± 0.01 | <0.0001 |
| Trehalose (g/100 g dw) | 2.71 ± 0.09 | 8.31 ± 0.01 | <0.0001 |
| Total sugars (g/100 g dw) | 17.09 ± 0.24 | 15.51 ± 0.14 | 0.0002 |
| C16:0 (relative percentage) | 11.23 ± 0.21 | 12.25 ± 0.25 | 0.0011 |
| C18:0 (relative percentage) | 1.79 ± 0.01 | 2.77 ± 00.04 | <0.0001 |
| C18:1n9 (relative percentage) | 38.61 ± 0.33 | 27.06 ± 0.09 | <0.0001 |
| C18:2n6 (relative percentage) | 43.49 ± 0.10 | 53.60 ± 0.37 | <0.0001 |
| SFA (relative percentage) | 16.94 ± 0.41 | 17.09 ± 0.31 | 0.4377 |
| MUFA (relative percentage) | 39.29 ± 0.31 | 28.59 ± 0.04 | <0.0001 |
| PUFA (relative percentage) | 43.77 ± 0.10 | 54.32 ± 0.27 | <0.0001 |
| α-Tocopherol (µg/100 g dw) | 12.48 ± 0.70 | 14.76 ± 1.13 | 0.0137 |
| β-Tocopherol (µg/100 g dw) | 12.88 ± 0.99 | 216.18 ± 0.90 | <0.0001 |
| γ-Tocopherol (µg/100 g dw) | nd | 296.67 ± 0.19 | 0.0032 |
| Total tocopherols (µg/100 g dw) | 25.36 ± 0.29 | 527.61 ± 2.22 | 0.0008 |
| Compounds | Leccinum molle (Bon) Bon | Leccinum vulpinum Watling | p-Value |
|---|---|---|---|
| Oxalic acid (g/100 g dw) | 0.29 ± 0.03 | 0.11 ± 0.01 | 0.0002 |
| Quinic acid (g/100 g dw) | nd | 0.13 ± 0.01 | <0.0001 |
| Citric acid (g/100 g dw) | 2.62 ± 0.16 | nd | <0.0001 |
| Fumaric acid (g/100 g dw) | 1.04 ± 0.02 | 0.25 ± 0.01 | <0.0001 |
| Total organic acids (g/100 g dw) | 3.95 ± 0.19 | 0.49 ± 0.01 | <0.0001 |
| Gallic acid (mg/100 g dw) | nd | 0.08 ± 0.01 | <0.0001 |
| Protocatechuic acid (mg/100 g dw) | nd | 0.35 ± 0.05 | <0.0001 |
| p-Hydroxybenzoic acid (mg/100 g dw) | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.0002 |
| Total phenolic acids (mg/100 g dw) | 0.06 ± 0.01 | 0.52 ± 0.05 | <0.0001 |
| Cinnamic acid (mg/100 g dw) | 0.13 ± 0.01 | 0.17 ± 0.01 | <0.0001 |
| Activity | Assay | Leccinum molle (Bon) Bon | Leccinum vulpinum Watling | p-Value |
|---|---|---|---|---|
| Reducing Power | Folin-Ciocalteu assay (mg GAE/g extract) | 17.76 ± 0.26 | 30.14 ± 0.70 | <0.001 |
| Ferricyanide/Prussian blue assay (EC50; mg/mL) | 2.13 ± 0.01 | 0.54 ± 0.01 | <0.001 | |
| Radical scavenging activity | DPPH radical-scavenging activity assay (EC50; mg/mL) | 10.68 ± 0.55 | 1.19 ± 0.02 | <0.001 |
| Lipid peroxidation inhibition | β-carotene/linoleate assay (EC50; mg/mL) | 2.23 ± 0.05 | 0.11 ± 0.01 | <0.001 |
| TBARS assay (EC50; mg/mL) | 1.48 ± 0.02 | 0.03 ± 0.00 | <0.001 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, F.S.; Barros, L.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Leccinum molle (Bon) Bon and Leccinum vulpinum Watling: The First Study of Their Nutritional and Antioxidant Potential. Molecules 2016, 21, 246. https://doi.org/10.3390/molecules21020246
Reis FS, Barros L, Martins A, Vasconcelos MH, Morales P, Ferreira ICFR. Leccinum molle (Bon) Bon and Leccinum vulpinum Watling: The First Study of Their Nutritional and Antioxidant Potential. Molecules. 2016; 21(2):246. https://doi.org/10.3390/molecules21020246
Chicago/Turabian StyleReis, Filipa S., Lillian Barros, Anabela Martins, M. Helena Vasconcelos, Patricia Morales, and Isabel C. F. R. Ferreira. 2016. "Leccinum molle (Bon) Bon and Leccinum vulpinum Watling: The First Study of Their Nutritional and Antioxidant Potential" Molecules 21, no. 2: 246. https://doi.org/10.3390/molecules21020246
APA StyleReis, F. S., Barros, L., Martins, A., Vasconcelos, M. H., Morales, P., & Ferreira, I. C. F. R. (2016). Leccinum molle (Bon) Bon and Leccinum vulpinum Watling: The First Study of Their Nutritional and Antioxidant Potential. Molecules, 21(2), 246. https://doi.org/10.3390/molecules21020246