Functional Characterization of a Dihydroflavanol 4-Reductase from the Fiber of Upland Cotton (Gossypium hirsutum)
Abstract
:1. Introduction

2. Results and Discussion
2.1. Cloning of DFR Gene from Fiber of G. hirsutum
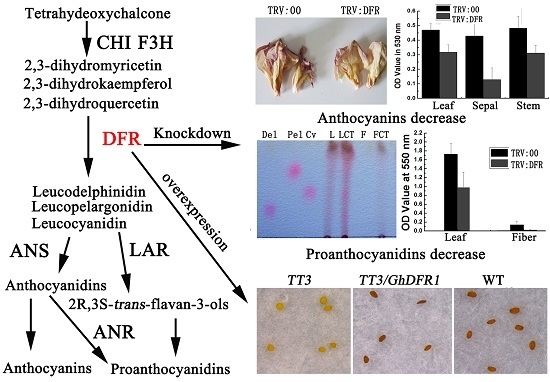
2.2. Silencing GhDFR1 Affected Flower Coloration and Content of Anthocyanins in Cotton Plants by VIGS
2.3. Silencing GhDFR1 Affected Structure and Content of PAs and Flavan-3-ols in Cotton Plants by VIGS





2.4. Overexpression of GhDFR1 Gene in Arabidopsis thaliana TT3 Mutant

2.5. Discussion
3. Experimental Section
3.1. Plant Materials
3.2. Total RNA Extract, Semi-RT-PCR, and qPCR
3.3. Cloning of GhDFR1 from G. hirsutum
3.4. VIGS Mediated by Agrobacterium
3.5. Extraction and Assay of Anthocyanins
3.6. Extraction of the Soluble PAs
3.7. PAs Hydrolysis and TLC Assay
3.8. LC-MS Analysis of Flavan-3-ols
3.9. Overexpression of GhDFR1 in A. thaliana TT3 Mutant
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, D.Y.; Jackson, L.A.; Cooper, J.D.; Ferreira, D.; Paiva, N.L. Molecular and biochemical analysis of two cdna clones encoding dihydroflavonol-4-reductase from medicago truncatula. Plant Physiol. 2004, 134, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Heidmann, I.; Forkmann, G.; Saedler, H. A new petunia flower color generated by transformation of a mutant with a maize gene. Nature 1987, 330, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Ryu, S.; Yi, H.K.; Shin, B.; Cheong, H.; Choi, G. Alteration of a single amino acid changes the substrate specificity of dihydroflavonol 4-reductase. Plant J. 2001, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Yi, H.; Shin, B.; Oh, B.J.; Cheong, H.; Choi, G. Cymbidium hybrida dihydroflavonol 4-reductase does not efficiently reduce dihydrokaempferol to produce orange pelargonidin-type anthocyanins. Plant J. 1999, 19, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, K.N.; Rohde, W. Structure of the hordeum-vulgare gene encoding dihydroflavonol-4-reductase and molecular analysis of ant18 mutants blocked in flavonoid synthesis. Mol. Genet. Genom. 1991, 230, 49–59. [Google Scholar] [CrossRef]
- Shirley, B.W.; Hanley, S.; Goodman, H.M. Effects of ionizing radiation on a plant genome: Analysis of two arabidopsis transparent testa mutations. Plant Cell 1992, 4, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Bonguebartelsman, M.; Oneill, S.D.; Tong, Y.S.; Yoder, J.I. Characterization of the gene encoding dihydroflavonol 4-reductase in tomato. Gene 1994, 138, 153–157. [Google Scholar] [CrossRef]
- Gosch, C.; Nagesh, K.M.; Thill, J.; Miosic, S.; Plaschil, S.; Milosevic, M.; Olbricht, K.; Ejaz, S.; Rompel, A.; Stich, K.; et al. Isolation of dihydroflavonol 4-reductase cdna clones from Angelonia x angustifolia and heterologous expression as gst fusion protein in Escherichia coli. PLoS ONE 2014, 9, e107755. [Google Scholar] [CrossRef] [PubMed]
- Miosic, S.; Thill, J.; Milosevic, M.; Gosch, C.; Pober, S.; Molitor, C.; Ejaz, S.; Rompel, A.; Stich, K.; Halbwirth, H. Dihydroflavonol 4-reductase genes encode enzymes with contrasting substrate specificity and show divergent gene expression profiles in fragaria species. PLoS ONE 2014, 9, e112707. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712. [Google Scholar] [CrossRef]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Z.; Zhu, Y.; Liu, Z.; Du, C.; Li, K.G.; Xie, D.Y. An integrated approach to demonstrating the anr pathway of proanthocyanidin biosynthesis in plants. Planta 2012, 236, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lee, S.S. Proanthocyanidins from the leaves of machilus philippinensis. J. Nat. Prod. 2010, 73, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Mole, S. The systematic distribution of tannins in the leaves of angiosperms—A tool for ecological-studies. Biochem. Syst. Ecol. 1993, 21, 833–846. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Waters, E.J.; Cheynier, V.; Herderich, M.J.; Vidal, S. Characterization of proanthocyanidins in grape seeds using electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D. Two a-type proanthocyanidins from prunus armeniaca roots. Fitoterapia 2000, 71, 245–253. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Mellway, R.D.; Tran, L.T.; Prouse, M.B.; Campbell, M.M.; Constabel, C.P. The wound-, pathogen-, and ultraviolet B-responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol. 2009, 150, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny revisited: Condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Pilu, R.; Cassani, E.; Sirizzotti, A.; Petroni, K.; Tonelli, C. Effect of flavonoid pigments on the accumulation of fumonisin b1 in the maize kernel. J. Appl. Genet. 2011, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Pandey-Rai, S. Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of artemisia annua l. Against short-term uv-b radiation. Planta 2014, 240, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Hagenbucher, S.; Olson, D.M.; Ruberson, J.R.; Wackers, F.L.; Romeis, J. Resistance mechanisms against arthropod herbivores in cotton and their interactions with natural enemies. Crit. Rev. Plant Sci. 2013, 32, 458–482. [Google Scholar] [CrossRef]
- Mascarenhas, R.N.; Boethel, D.J.; Leonard, B.R.; Boyd, M.L.; Clemens, C.G. Resistance monitoring to Bacillus thuringiensis insecticides for soybean loopers (Lepidoptera: Noctuidae) collected from soybean and transgenic bt-cotton. J. Econ. Entomol. 1998, 91, 1044–1050. [Google Scholar] [CrossRef]
- Smith, C.W.; Mccarty, J.C.; Altamarino, T.P.; Lege, K.E.; Schuster, M.F.; Phillips, J.R.; Lopez, J.D. Condensed tannins in cotton and bollworm-budworm (Lepidoptera: Noctuidae) resistance. J. Econ. Entomol. 1992, 85, 2211–2217. [Google Scholar] [CrossRef]
- Hanny, B.W. Gossypol, flavonoid, and condensed tannin content of cream and yellow anthers of 5 cotton (Gossypium hirsutum L.) cultivars. J. Agric. Food Chem. 1980, 28, 504–506. [Google Scholar] [CrossRef]
- Lane, H.C.; Schuster, M.F. Condensed tannins of cotton leaves. Phytochemistry 1981, 20, 425–427. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.Y.; Peng, Q.Z.; Tang, Y.T.; Xia, G.X.; Wu, J.H.; Xie, D.Y. Functional characterization of an anthocyanidin reductase gene from the fibers of upland cotton (Gossypium hirsutum). Planta 2015, 241, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Gene characterization, analysis of expression and in vitro synthesis of dihydroflavonol 4-reductase from [Citrus sinensis (L.) osbeck]. Phytochemistry 2006, 67, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Zhu, Y.; Li, Q.; Liu, J.Z.; Tian, Y.C.; Liu, Y.L.; Wu, J.H. Development of agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in gossypium barbadense. PLoS ONE 2013, 8, e73211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Peng, Q.Z.; Du, C.; Li, K.G.; Xie, D.Y. Characterization of flavan-3-ols and expression of myb and late pathway genes involved in proanthocyanidin biosynthesis in foliage of vitis bellula. Metabolites 2013, 3, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Yang, T.J.; Hur, Y.; Nou, I.S. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in brassica rapa. Gene 2014, 550, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bernays, E.A. Plant tannins and insect herbivores—An appraisal. Ecol. Entomol. 1981, 6, 353–360. [Google Scholar] [CrossRef]
- Mole, S.; Rogler, J.C.; Morell, C.J.; Butler, L.G. Herbivore growth reduction by tannins—Use of waldbauer ratio techniques and manipulation of salivary protein-production to elucidate mechanisms of action. Biochem. Syst. Ecol. 1990, 18, 183–197. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for agrobacterium-mediated transformation of arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, Y.; Wang, P.; Fan, Q.; Wu, Y.; Peng, Q.-Z.; Xia, G.-X.; Wu, J.-H. Functional Characterization of a Dihydroflavanol 4-Reductase from the Fiber of Upland Cotton (Gossypium hirsutum). Molecules 2016, 21, 32. https://doi.org/10.3390/molecules21020032
Wang L, Zhu Y, Wang P, Fan Q, Wu Y, Peng Q-Z, Xia G-X, Wu J-H. Functional Characterization of a Dihydroflavanol 4-Reductase from the Fiber of Upland Cotton (Gossypium hirsutum). Molecules. 2016; 21(2):32. https://doi.org/10.3390/molecules21020032
Chicago/Turabian StyleWang, Le, Yue Zhu, Peng Wang, Qiang Fan, Yao Wu, Qing-Zhong Peng, Gui-Xian Xia, and Jia-He Wu. 2016. "Functional Characterization of a Dihydroflavanol 4-Reductase from the Fiber of Upland Cotton (Gossypium hirsutum)" Molecules 21, no. 2: 32. https://doi.org/10.3390/molecules21020032