Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Capacity and Total Phenolics, Anthocyanin and Flavonoids Contents
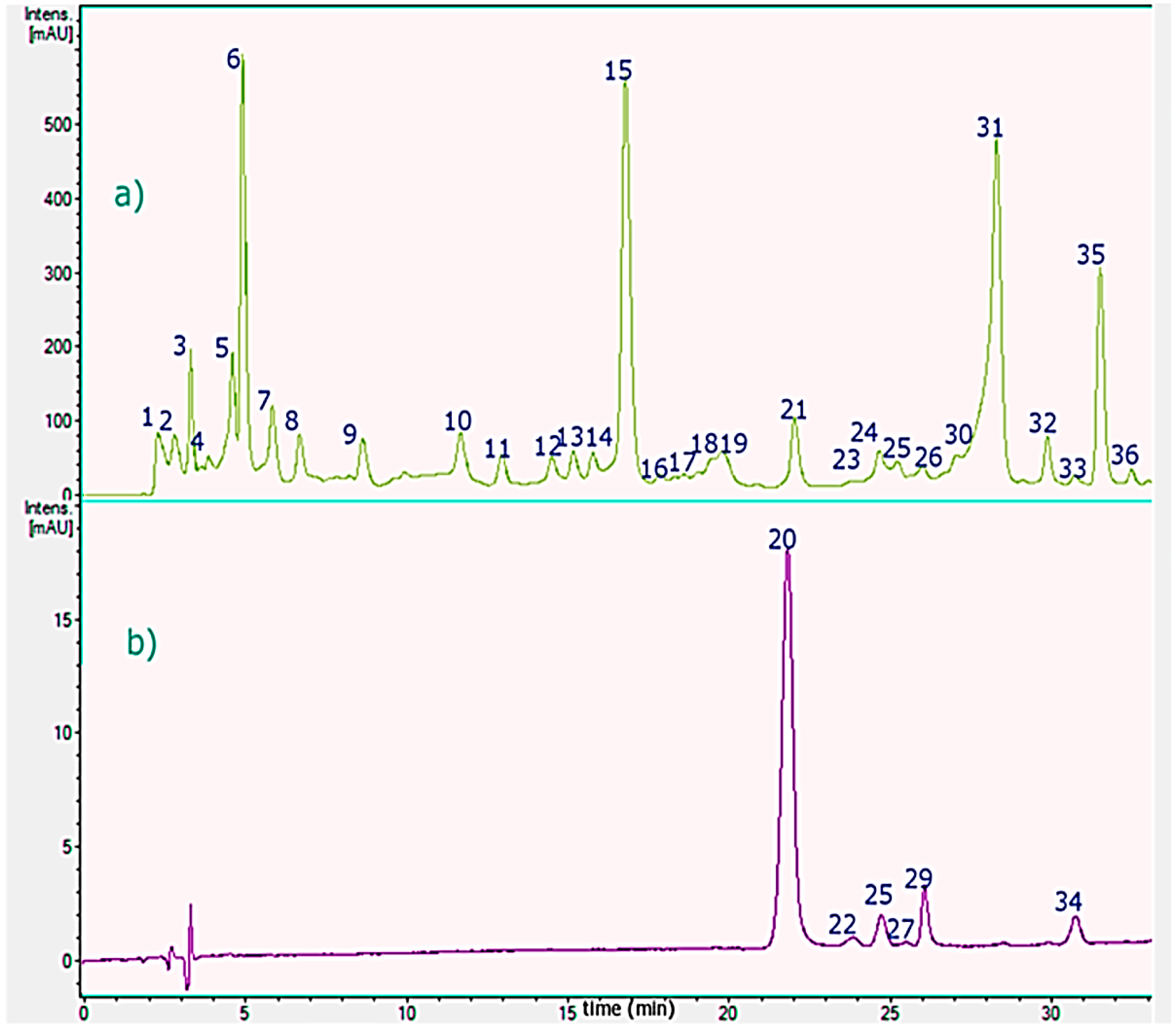
2.2. MS-PDA Identification of Phenolic Acids in Chilean Mistletoe (Lorantaceae) From Southern Chile
2.2.1. Flavonoids
2.2.2. Phenolic Acids
2.2.3. Oxylipins
2.2.4. Procyanidins
2.2.5. Anthocyanins
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Sample Preparation
3.3. Instrumentation
3.4. LC Parameters
3.5. MS Parameters
3.6. Antioxidant Assays
3.6.1. Ferric Reducing Antioxidant Power
3.6.2. Superoxide Anion Scavenging Activity
3.7. Polyphenol and Flavonoids Contents
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burgos, A.N.; Morales, M.A. Qualitative study of use medicinal plants in a complementary or alternative way with the use of among of rural population of the Bulnes City, Bío-Bío Region, Chile. BLCPMA 2010, 9, 377–387. [Google Scholar]
- De Mösbach, E.W. Botánica Indígena de Chile. In Museo Chileno de Arte Precolombino, Fundación Andes y Editorial Andrés Bello; Aldunate, C., Villagrán Santiago, C., Eds.; Andres Bello: Santiago, Chile, 1991; pp. 95–96. [Google Scholar]
- Hoffmann, A.; Arroyo, M.T.K.; Liberona, F.; Muñoz, M.; Watson, J.M. Plantas Altoandinas en la Flora Silvestre de Chile; ediciones Fundación Claudio A. Gay: Santiago de Chile, Chile, 2000. [Google Scholar]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Sci. Food Agric. 2009, 57, 5271–5278. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Silva, M.; Becerra, J.; Schmeda-Hirschmann, G. Direct characterisation of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS). Food Chem. 2012, 131, 318–327. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A. Flavonol Regulation in Tumor Cells. J. Cell. Biochem. 2015, 116, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, A.; Chen, E.; Singh, R.K.; Chichester, C.O.; Moore, R.G.; Singh, A.P.; Vorsa, N. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int. J. Oncol. 2015, 46, 1924–1934. [Google Scholar] [PubMed]
- Steinmann, D.; Ganzera, M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 2011, 55, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Wright, P. Metabolite identification by mass spectrometry: Forty years of evolution. Xenobiotica 2011, 41, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Mattoli, L.; Cangi, F.; Ghiara, C.; Burico, M.; Maidecchi, A.; Bianchi, E.; Ragazzi, E.; Bellotto, L.; Seraglia, R.; Traldi, P. A metabolite fingerprinting for the characterization of commercial botanical dietary supplements. Metabolomics 2011, 7, 437–445. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Kang, H.J.; Yang, H.J.; Kim, M.J.; Han, E.S.; Kim, H.J.; Kwon, D.Y. Metabolomic analysis of meju during fermentation by ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS). Food Chem. 2011, 127, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Domínguez, G.; Romero-González, R.; Garrido Frenich, A. Multi-class methodology to determine pesticides and mycotoxins in green tea and royal jelly supplements by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Food Chem. 2016, 197, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceuticals and personal-care products in environmental matrices. TrAC 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- He, D.X.; Shan, Y.; Wu, Y.H.; Liu, G.Z.; Chen, B.; Yao, S.Z. Simultaneous determination of flavanones, hydroxycinnamic acids and alkaloids in citrus fruits by HPLC-DAD-ESI/MS. Food Chem. 2011, 127, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Borquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequen. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Vallejos, J.; Areche, C.; Sepúlveda, B. Concise and Straightforward Asymmetric Synthesis of a Cyclic Natural Hydroxy-Amino Acid. Molecules 2014, 19, 19516–19531. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; López-Gresa, M.P.; Vilanova, S.; Torres, C.; Hurtado, M.; Gramazio, P.; Andújar, I.; Herráiz, F.J.; Bellés, J.M.; Prohens, J. Diversity and Relationships in Key Traits for Functional and Apparent Quality in a Collection of Eggplant: Fruit Phenolics Content, Antioxidant Activity, Polyphenol Oxidase Activity, and Browning. J. Agric. Food Chem. 2013, 61, 8871–8879. [Google Scholar] [CrossRef] [PubMed]
- López-Gresa, M.P.; Torres, C.; Campos, L.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; Conejero, V. Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ. Exp. Bot. 2011, 74, 216–228. [Google Scholar] [CrossRef]
- Hong, S.M.; Choi, J.-H.; Jo, S.-J.; Song, S.K.; Lee, J.M.; Kusakabe, T. Expression of Recombinant Viscum Album Coloratum lectin B-chain in the Silkworm Expression System and Evaluation of Antioxidant Activity. Biotech. Bioproc. Eng. 2015, 20, 515–522. [Google Scholar] [CrossRef]
- Orhan, D.D.; Senol, F.S.; Hosbas, S.; Orhan, I.E. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant properties of Viscum album L. samples collected from different host plants and its two principal substances. Ind.Crops Prod. 2014, 62, 341–349. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Raieviawati, S.I.; Ishmaru, K.; Hou, D.-X.; Hayashi, N. Antioxidant Activity and Phenolic Content of Mistletoe Extracts Following High-Temperature Batch Extraction. Food Sci. Technol. Res. 2014, 20, 201–206. [Google Scholar]
- Simirgiotis, M.J.; Benites, J.; Areche, A.; Sepulveda, B. Antioxidant capacities and analisis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Medicinal plants, chemical composition and quality: May blackcurrant buds and blackberry sprouts be a new polyphenol source for herbal preparations? J. Appl. Bot. Food Qual. 2013, 86, 79–89. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Ye, F.; Liang, Q.; Li, H.; Zhao, G. Solvent effects on phenolic content, composition, and antioxidant activity of extracts from florets of sunflower (Helianthus annuus L.). Ind. Crops Prod. 2015, 76, 574–581. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Se´púlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean berries extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Ye, M.; Yang, W.-Z.; Liu, K.-D.; Qiao, X.; Li, B.-J.; Cheng, J.; Feng, J.; Guo, D.-A.; Zhao, Y.-Y. Characterization of flavonoids in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MSn. J. Pharm. Anal. 2012, 2, 35–42. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; de Beer, D.; Stander, M.A.; Joubert, E. Comprehensive phenolic profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS reveals novel xanthone and benzophenone constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Theoduloz, C.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Comparison of phenolic composition and antioxidant properties of two native Chilean and one domestic strawberry genotypes. Food Chem. 2009, 113, 377–385. [Google Scholar] [CrossRef]
- Bensouici, C.; Kabouche, A.; Karioti, A.; Ozturk, M.; Duru, M.E.; Bilia, A.R.; Kabouche, Z. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharm. Biol. 2016, 54, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spect. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography-UV detection-mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef] [PubMed]





| Species | DPPH− a | FRAP b | SAA c | TPC d | TFC e | TAC f | Extraction Yields (%) g |
|---|---|---|---|---|---|---|---|
| T. tetrandus leaves | 13.38 ± 0.47 | 125.32 ± 5.96 | 84.06 ± 4.59 | 37.34 ± 0.92 | 26.77 ± 2.76 | - | 10.16 |
| T. tetrandus flowers | 23.40 ± 0.40 | 85.32 ± 3.22 | 57.24 ± 4.36 b | 24.60 ± 1.12 | 17.54 ± 1.75 | 17.32 ± 1.42 | 8.83 |
| Gallic acid h | 1.47 ± 0.05 (7.99 ± 0.99 mM) | 143.2 ± 6.67 | 97.55 ± 1.53 | - | - | - | - |
| Quercetin h | 9.69 ± 0.19 (32.01 ± 0.62 mM) | 91.12 ± 5.27 | 61.72 ± 1.18 c | - | - | - | - |
| Cyanidin 3-O-glucoside h | 28.67 ± 0.22 (59.23 ± 0.47 mM) | 82.19 ± 4.87 | 56.48 ± 1.06 b,c | - | - | - | - |
| Peak # | Uv Max | Tentative Identification | Molecular Formula | Retention Time (min) | Theoretical Mass (m/z) | Measured Mass (m/z) | Other Ions (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | - | Quinic acid | C7H11O6− | 2.1 | 191.0561 | 191.0551 | - |
| 2 | 325 | Caffeoyl-glucose | C15H17O9− | 2.3 | 341.0878 | 341.0870 | 191.0555 (Quinic acid C7H11O6−) |
| 3 | 236, 326 | 3-O-caffeoylquinic acid (3-CQA) * | C16H17O9− | 3.0 | 353.0878 | 353.0873 | 707.1825 [2M − H]−, 191.0552 |
| 4 | 236, 326 | Caffeoyl-shikimic acid | C16H15O8- | 3.2 | 335.0532 | 335.0766 | |
| 5 | 275 | p-Coumaric acid | C16H17O9− | 4.1 | 163.0401 | 163.0390 | |
| 6 | 280 | (+)-Catechin * | C15H13O6− | 4.6 | 289.07176 | 289.0715 | |
| 7 | 254, 354 | Rutin * | C27H29O16− | 4.9 | 609.1461 | 609.1449 | 463.0870, 301.0344 (Quercetin) |
| 8 | 280 | Epigallocatechin | C15H13O7− | 5.5 | 305.0667 | 305.0304 | |
| 9 | 240, 325 | Feruloyl quinic acid (3-FQA) | C17H19O9− | 6.2 | 367.1034 | 367.1028 | |
| 10 | 280 | Gallocatechin | C15H13O7− | 8.5 | 305.0667 | 305.0301 | |
| 11 | 254, 354 | Quercetin-3-O-glucose * | C21H19O12− | 12.2 | 463.0882 | 463.0877 | 300.0276 (Quercetin C15H9O7−) |
| 12 | 254, 354 | Quercetin-3-O-pentose * | C20H17O11− | 12.5 | 433.1033 | 433.2019 | 300.0276 (Quercetin C15H9O7−) |
| 13 | 254, 354 | Apiin | C26H27O14− | 14.4 | 563.1406 | 563.1382 | |
| 14 | 254, 354 | p-Coumaroyl malate | C13H11O7− | 15.3 | 279.0510 | 279.0507 | |
| 15 | 254, 354 | Quercetin-3-O-glucosyl-derivative | C27H23O16− | 16.5 | 615.0843 | 615.0983 | |
| 16 | 236, 329 | di-O-Caffeoylquinic acid (di-CQA) | C25H23O12− | 17.2 | 515.1195 | 515.1192 | |
| 17 | 236, 326 | 5-O-Caffeoylquinic acid (3-CQA) * | - | 18.0 | 353.0878 | 353.0876 | |
| 18 | - | Oxylipin (trihydroxyoctadecadienoic acid) | C18H31O5− | 19.2 | 327.2146 | 327.2144 | |
| 19 | 260 | Oxylipin (trihydroxyoctadecaenoic acid) | C18H33O5− | 22.0 | 329.2147 | 329.2322 | |
| 20 | 254, 354 | Delphinidin-3-O-glucose | C21H21O12+ | 22.2 | 465.1033 | 465.1104 | 303.0162 (Delphinidin) |
| 21 | Naringenin-7-O-glucoside | C21H21O10− | 24.9 | ||||
| 22 | 254, 354 | Cyanidin-3-O-glucose | C21H21O11+ | 24.8 | 449.1135 | 449.1149 | 287.0567 (Cyanidin) |
| 23 | 254, 354 | Isorhamnetin-3-O-galactose | C22H21O12− | 25.0 | 477.1038 | 477.1031 | |
| 24 | 236, 329 | di-O-Caffeoylquinic acid (di-CQA) | C25H23O12− | 25.2 | 515.1195 | 515.1189 | |
| 25 | 254, 354 | Malvidin * | C17H15O7− | 25.4 | 331.0123 | 331.0117 | |
| 26 | 254, 354 | 7-O-Methylisorhamnetin | C17H13O7− | 25.6 | 329.0627 | 329.0670 | |
| 27 | 254, 354 | Pelargonidin | C15H11O5+ | 25.8 | 271.0606 | 271.0618 | |
| 28 | 254, 354 | Isorhamnetin-3-O-glucose | C22H21O12− | 26.1 | 477.1038 | 477.1031 | |
| 29 | 254, 354 | Delphinidin | C15H11O7− | 26.4 | 303.0505 | 303.0161 | |
| 30 | - | Phenyllactic acid hexoside | C15H17O5− | 26.9 | 327.2178 | 327.2178 | |
| 31 | 254, 354 | Isorhamnetin | C16H11O7− | 28.5 | 315.0238 | 315.0499 | |
| 32 | 254, 354 | Quercetin * | C15H9O7− | 29.9 | 301.0264 | 301,0353 | |
| 33 | 254, 347 | Luteolin * | C15H9O6− | 30.2 | 285.0405 | 285.0392 | |
| 34 | 254, 354 | Cyanidin | C15H11O6+ | 31.0 | 287.0556 | 287.0569 | |
| 35 | 292. 330sh | Naringenin * | C15H12O5− | 32.5 | 271.0824 | 271.0610 | |
| 36 | 254, 347 | Apigenin * | C15H9O5− | 34.0 | 269.0455 | 269.0443 |
- Sample Availability: Samples of the plant material and extracts are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules 2016, 21, 245. https://doi.org/10.3390/molecules21030245
Simirgiotis MJ, Quispe C, Areche C, Sepúlveda B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules. 2016; 21(3):245. https://doi.org/10.3390/molecules21030245
Chicago/Turabian StyleSimirgiotis, Mario J., Cristina Quispe, Carlos Areche, and Beatriz Sepúlveda. 2016. "Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties" Molecules 21, no. 3: 245. https://doi.org/10.3390/molecules21030245
APA StyleSimirgiotis, M. J., Quispe, C., Areche, C., & Sepúlveda, B. (2016). Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules, 21(3), 245. https://doi.org/10.3390/molecules21030245









