1. Introduction
1.1. Metalloporphyrins and Homogeneous Catalysis
Porphyrins are highly conjugated heterocyclic macrocycles derived from four pyrrole rings interconnected by four methine bridges [
1]. The chemical and physical properties of these compounds allow for their use in various fields [
1,
2]. In fact, porphyrins have promising medicinal [
3] and catalytic [
4] applications.
Porphyrin macrocycles can coordinate different catalytically active metals, like iron, copper, and nickel, among others. The catalytic use of the resulting metal complex will depend on the selected metal. Metalloporphyrins (MPs) occur in the nuclei of several oxidation enzymes such as cytochromes P450 [
5,
6], peroxidases [
6], and catalases; they are often employed as model catalysts [
7].
The great versatility of reactions catalyzed by P450 biomimetic systems; e.g., alkane hydroxylation and olefin epoxidation, regio- and stereo-selective reactions, and drug metabolism, has motivated researchers to develop new porphyrin derivatives [
8,
9].
Groves [
10] performed the pioneered studies on MPs as catalysts and showed that the Fe
3+ complex [Fe(TPP)Cl] can mimic cytochrome P450 in many reactions, mainly hydroxylation and epoxidation, which boosted research in this field [
4,
5,
8].
MPs can efficiently and selectively catalyze a series of oxidation reactions in homogeneous medium (
i.e., when the catalyst, substrate, and products are in the same phase). However, depending on the porphyrin structure, secondary reactions (MP destructive oxidation by other previously activated MP and secondary MP–MP interaction reaction; e.g., MP dimerization by μ-oxo bridges) may take place in homogeneous medium, to deactivate the catalytic species [
11,
12]. Moreover, homogeneous systems preclude catalyst recovery and reuse [
13,
14].
To overcome these common issues in homogeneous medium, various research groups have attempted to design catalysts for heterogeneous processes by immobilizing MPs on inorganic or organic solid supports [
11,
15,
16,
17,
18]. MP immobilization should facilitate catalyst recovery and reuse as well as minimize destructive oxidation and other deactivating processes, ultimately preventing catalyst efficiency loss.
A variety of solid matrixes can serve as supports for MPs; for example, silicas, zeolites, polymeric nanoparticles, dendrimers, natural and synthetic layered hydroxides, and fibrous compounds, among others.
1.2. Metalloporphyrins and Heterogeneous Catalysis
In 1983, van der Made and co-workers [
15] published one of the first reports on MP immobilization with a view to stabilizing the MP complex and promoting catalyst recycling; the catalyst support consisted of a polymeric matrix. These researchers realized that isolating the MP active site by immobilizing a MnP on a polymer support (isocyanide) was possible and avoided formation of the less reactive dimers observed during homogeneous catalysis. In fact, the immobilized MnP was three times more active for cyclohexene epoxidation than the free MnP [
15].
Since then, a large number of inorganic solids have been studied as supports for MPs and many other complexes for catalytic purposes. Examples of these solids include clay minerals [
19,
20,
21], silica [
22,
23], and metal oxides [
24,
25], among others [
26]. Besides, polymeric matrixes like isocyanide [
15], poly(vinyl alcohol) [
27], and polystyrene [
28] have also been employed to immobilize MPs and obtain efficient catalysts for oxidation reactions.
MP immobilization can also avoid the undesired approach between catalytic species and the consequent catalyst destruction. Moreover, proper combination of MP and support mimics the protein cavity of the natural enzyme and modulates specific selectivity for various catalytic reactions [
19,
29].
Facile catalyst recovery from the heterogeneous catalytic reaction solution is among the advantages of catalyst immobilization and enables catalyst recycling. Catalyst recovery often demands just simple filtration or centrifugation [
11,
19].
In this context, layered double hydroxides (LDHs) and layered hydroxide salts (LHSs) have emerged as interesting supports to immobilize MPs. These matrixes are easy to obtain by one-pot synthesis, and their variable chemical composition is based on environmentally friendly elements. Additionally, they have layered morphology, controllable particle size, inertia in different experimental conditions, and low cost.
Layered compounds can interact with MPs not only at the crystal basal surface, but also at the crystal edge. MPs can even intercalate between the matrix layers. All these alternatives expand the applicability of this class of compounds. When MPs intercalate between LDH or LHS layers [
30,
31,
32], the resulting solid displays distinct and unusual selectivity as compared to the catalyst in solution (homogeneous catalysis) or even to the MP immobilized on the layered compound surface [
33,
34].
This short review offers a brief overview of the articles about MP immobilization on two different layered compounds, LDHs and LHSs, published over the past years. It also includes new data for an MP immobilized on different Mg/Al-LDHs.
1.3. Layered Compounds
Layered compounds are part of an unusual class of compounds obtained by packing of two-dimensional units (layers) along the basal crystallographic axis. These compounds have called researchers’ attention over the last years mainly because it is possible manipulate single layers, but this manipulation is not possible on the traditional tridimensional materials.
Depending on the layer charges, layered compounds of synthetic or natural origin can be classified into neutral, positive, or negative. These charges will depend on the material that is present during the genesis or the oxi-reduction process in which the metal cation undergoes oxidation or reduction, to intercalate cations or anions, respectively [
35]. In some examples, neutral species can also intercalate between the layers by just breaking the bonds that hold the layers together (e.g., kaolinite intercalated with different neutral molecules).
The layer thickness will depend on the material used to prepare the layered compound, and the material may be one-, three-, or seven-atom-thick as in the case of graphite, transition metal dichalcogenides, and 2:1 cationic exchange clay minerals or micas, respectively.
Anionic Exchange layered compounds are rare in nature, but it is possible to obtain countless compounds of this type in the laboratory by using simple synthetic procedures. Most of these compounds are based on the Brucite structure (Mg(OH)2), which is common for calcium, manganese, iron, cobalt, and nickel hydroxides, all of which bear a double positive charge.
The Brucite structure consists of layers of slightly distorted octahedra. In the center of these octahedral Mg
2+, cations are coordinated with six hydroxyl groups occupying the vertices of the octahedra. In turn, each hydroxyl group coordinates with three Mg
2+ cations, to generate a tri-octahedral structure where all the octahedral sites of the structure are filled. The octahedral unit shares vertices with three adjacent octahedra connected by the corners (
Figure 1) the 2+ charge of the Mg
2+ cation is shared by six bonds with the hydroxyls, to give a charge of (1/3)+. Because three Mg
2+ bonds share the single charge on hydroxyl, the result is (1/3)−. Therefore, the (1/3)+ and (1/3)− charges will cancel each other out [
36,
37].
Another common example of simple hydroxide is Gibbsite (Al(OH)3). Because Gibbsite contains Al3+ in its structure, one third of the octahedral positions remain unoccupied, to ensure structural neutrality. This arrangement gives rise to the structure called di-octahedral. Charge balance for these structures is based on the Pauling electrostatic valence theory, which postulates that each cation present in the polyhedra tends to compensate the electric charge of each anion in stable coordinated structures. These layers bind to each other by Van der Waals forces. This interaction furnishes three-dimensional crystals that tend to grow preferentially along the layer planes, to afford crystals with plaque morphology.
The majority of layered compounds occurring in the mineral form have neutral structure or constitute cationic exchangers; e.g., clay minerals belonging to the 2:1 group. However, layered minerals with anionic exchange properties are rare. Examples of such minerals are hydrotalcite-like compounds, also known as layered double hydroxides (LDHs).
The LDH structure resembles the structure of the mineral Brucite, in which trivalent cations can isomorphically substitute the Mg2+ cations, to generate a positively charged residue. The presence of anions in the LDH interlayer space compensates for this charge and renders these compounds their characteristic anionic exchange capacity.
The formula [M
2+1−xM
3+x(OH)
2]
x+·A
m−x/m·nH
2O represents the LDH composition, where M
2+ refers to a divalent metallic cation (e.g., Mg
2+, Zn
2+,
etc.), M
3+ corresponds to a trivalent metallic cation (e.g., Al
3+, Fe
3+,
etc.), and A
m− represents an intercalated hydrated anion with charge m
− (e.g., NO
3−, CO
32−,
etc.). Although LDH are rare in nature, synthesizing these compounds in the laboratory is easy and relatively inexpensive [
38,
39,
40].
To be part of the LDH structure, the metallic cations M2+ and M3+ must remain in the center of a hydroxyl-coordinated octahedron, which implies in a limited range of ionic radii. In general, the molar ratio between the di- and trivalent metallic cations in the LDH (M2+/M3+) can vary from 1 to 8, which corresponds to 0.5 > x > 0.11 in the general LDH formula. Because each trivalent cation accounts for the excess positive charge in the layers, the x value determines the LDH charge density.
Higher charge density in the layer increases the amount of intercalated anions for each divalent cation, an equivalent amount of anions should intercalate between the LDH layers. Hence, increased M2+/M3+ molar ratio lowers the charge density and the number of intercalated anions. Consequently, the anions become sufficiently separated, which minimizes interaction between the pillared layers and facilitates delamination and exfoliation. Intercalated anions can be organic or polymeric, for example, which aids tailoring of the LDH properties to the desired application. When a low proportion of organic species intercalates between the layers, neutral organic species can occupy the spaces available between the organic anions, to cause a phenomenon called adsolubilization.
Figure 2 shows a schematic representation of an LDH structure of the 3R polytype (three layers along the basal axis) according to the nomenclature of Ramsdell.
Some literature reports have mentioned that M4+ cations can also substitute M2+ metals in LDHs, but the literature lacks unequivocal confirmation of these structures.
A distinct example of LDH is the structure of Gibbsite (Al(OH)
3). This compound reacts with excess lithium salts to form compounds similar to LDH of the type Li
0.5Al(OH)
3(A)
0.5·yH
2O or [LiAl
2(OH)
6]A·zH
2O (A = Cl
−, Br
−, NO
3−,
etc.) [
41]. These compounds are impossible to be synthesized by the regular precipitation route. Indeed, only the reaction of Gibbsite with lithium salts, either in solution or after mechanochemical reaction, yields these compounds. The lithium cations most likely occupy an octahedral site of the Gibbsite structure (Gibbsite is a di-octahedral structure where one octahedral site of the layers is empty), and the hydrated anion intercalates in the interlayer space (
Figure 3). Besides Gibbsite, the same type of reaction can also occur for a mixture of Nordstrandite and Bayerite, which bear similar structures [
42].
Specific literature review papers exist for the majority of these layered compounds, but a class of layered materials with anionic exchange capacity, denominated layered hydroxide salts (LHSs) and which are rare as mineral, will also be the focus of the present review article.
LHSs are compounds that resemble LDHs, but another mechanism rather than M
2+ substitution with M
3+ generates the excess positive charge in its layers. The general formulation of LHSs is M
2+(OH)
2−x(A
n−)
x/n·yH
2O, where M
2+ is a metallic cation (e.g., Ni
2+ Zn
2+, Ca
2+, Cd
2+, Co
2+, or Cu
2+), and A is a counter anion with charge n
−. (Ex.: NO
3−, SO
42−, CH
3COO
−,
etc. [
43,
44]).
The cationic layers in the LHS and the LDH structures have distinct generation mechanism. In LHSs, other anions occupy a fraction of the hydroxide sites, as in the case of Cu
2(OH)
3NO
3. Two different polymorphs exist in this phase. One polymorph is orthorhombic, as reported for the structure of Gerhardtite and other synthetic monoclinic compounds. In the orthorhombic structure of Cu
2(OH)
3NO
3, nitrate anions substitute one fourth of the hydroxide anions and coordinate with three different Cu
2+ cations, to occupy octahedral sites (
Figure 4A). Although nitrate anions are grafted to the layers, they can be replaced by means of anion exchange reactions. In the case of the second polymorph, one fourth of the Zn
2+ ions present in the octahedral sites are removed from the structure, and two tetrahedral sites are generated in the upper and lower octahedral empty site. The anions are grafted directly to the tetrahedral Zn
+2 sites, as observed for the mineral Simonkolleite (Zn
5(OH)
8Cl
2·2H
2O) (
Figure 4B). A third polymorph in which a structure similar to Simonkolleite contains water molecules directly bound to the tetrahedral Zn
2+ sites is also possible, but it requires the presence of a counter anion in the second coordination sphere, to neutralize the positive charges of the layers (e.g., Zn
5(OH)
8(NO
3)
2·2H
2O) (
Figure 4C).
The structure of the aforementioned zinc hydroxide nitrate Zn5(OH)8(NO3)2·2H2O can be considered a structural variation of the Zn(OH)2 structure, where one fourth of the octahedral Zn2+ cations are removed from the layer. Every octahedral site occupied by a Zn2+ cation shares edges with two empty and four occupied octahedra, producing a layer with a residual negative charge ([Zn3(OH)8]2−). To compensate for the deficit in positive charge, tetrahedral Zn2+ cations accommodate below and above the empty octahedra of the layer. In this way, three corners of the tetrahedra coordinate with oxygen atoms of the layer, whereas the fourth position coordinates with a water molecule. In this configuration, the layer is positively charged [Zn3(oct.)(OH)8Zn2(tet.)(H2O)2]2+, where “oct.” and “tet.” represent the Zn2+ cations located in octahedral and tetrahedral sites, respectively. To neutralize the residual positive charge of the layer, anions intercalate between the layers. Nitrate anions intercalate between the layers in the case discussed here.
Natural minerals contain more than one metal, which has led to identification of several layered hydroxide slats containing two different divalent metals. The resulting compounds are denominated double hydroxide salts (DHSs). They have the generic formula M
a1−xM
bx(OH)
2−x(A
n−)
x/n·yH
2O, where M
a and M
b are two different divalent cations, and A is the counter anion with charge n
−. Aurichalcite ((Zn,Cu)
5(CO
3)
2(OH)
6), Haydeeite (Cu
3Mg(OH)
6Cl
2) and Kapelassite (Cu
3Zn(OH)
6Cl
2), among others [
45], are examples of these structures.
Less frequently, layered hydroxide salts with three different metals have been reported; e.g., Loseyite (Mn,Zn,Mg)
4Zn
3(CO
3)
2(OH)
10 or Sclarite (Mn,Zn,Mg)
4Zn
3(CO
3)
2(OH)
10 [
46,
47].
3. Influence of the LDH Composition on the MnP Immobilization Rates and Catalytic Performance: New Results
Motivated by the rich chemistry of MP immobilization on layered compounds and its implication for the efficiency and selectivity of the obtained solids, our research group has studied how the LDH composition impacts the immobilization rates and catalytic performance in cyclooctene oxidation of [5,10,15,20-tetrakis(2,6-difluoro-3-sulfonatephenyl porphyrinate) manganese(III)] acetate (MnP) (the parent FeP is illustrated in
Figure 6).
First, we synthesized different LDH solids by obtaining LDHs from magnesium or zinc salts (M
2+) and aluminum (M
3+) at different nominal molar ratios (M
2+/M
3+ = 2:1, 3:1 or 4:1), with different interlayer anions (CO
32− and NO
3−). The co-precipitation method with increasing pH yielded LDHs containing intercalated carbonate or nitrate [
93].
Table 2 shows the nomenclature used for the obtained solids.
X-ray powder diffraction (
Figure 7) aided characterization of the solids. The calculated basal distances agreed with literature data on this class of compounds and were typical of an LDH solid bearing intercalated carbonate (~7.65 Å) (
Figure 7a) or nitrate (~8.79 Å) (
Figure 7b) ions [
94,
95,
96]. Recently, the structure of LDHs have been obtained also by X-ray [
97] or electron diffraction methods [
98].
The cell parameters “a” referred to the distance between the metal ions present in the LDH layer.
Table 2 lists the values for this parameter and revealed an increasing trend higher M
2+/M
3+ ratio increased the ionic radius of each M
2+ (the ionic radius of Mg
2+ and Zn
2+ is 0.74 Ǻ and 0.66 Ǻ, respectively).
Infrared analyses of the synthesized LDHs (
Supplementary Data, Figure S1) evidenced the typical vibrational bands profile of these materials. The band at 3434 cm
−1 was related to stretching of the O–H bond in the hydroxyl groups of the layers and the co-intercalated water molecules. The band at 1625 cm
−1 was due to angular deformation of water molecules. The bands in the region below 600 cm
−1 were characteristic of metal-oxygen (M–O) stretching. The spectra of the LDHX solids (X = 1–5) displayed the typical bands of carbonate ions at 1366 cm
−1 and 1636 cm
−1. The solids also featured a band at 1382 cm
−1, characteristic of nitrate ions, which was more pronounced in the spectrum of LDH4. This small contamination with nitrate ions probably resulted from the starting chemicals (nitrate salts).
In the spectra of the LDHX solids (X = 6–11), bands ascribed to nitrate ion appeared at 1382 cm
−1 and 1023 cm
−1. In the same spectra, a shoulder at 1366 cm
−1 indicated that these compounds also contained traces of carbonate ions [
94,
96].
Thermogravimetric analysis (TGA) helped to estimate the water content (physisorbed and intercalated) and the chemical composition of the solids based on the residual oxides content (
Supplementary Data, Figure S2) (
Table 3) [
99].
Table 4 summarizes the solids resulting from MnP immobilization on the different LDH solids (named MnP-LDHX, where MnP refers to [Mn(TDFSPP)] and X refers to the prepared LDH).
Figure 8 brings the UVVIS results obtained for the prepared solids.
Figure 8b depicts the UVVIS spectrum (in Nujol mull) of immobilized MnP-LDH3. The spectra displayed the typical MnP Soret band near 463 nm [
84], confirming MnP immobilization on the solid. Lack of changes in the basal distance, as revealed by X-ray analysis (data not shown), indicated MnP surface immobilization and excluded MnP intercalation between the LDH layers. All the prepared solids presented the same behavior.
Figure 8a depicts the UVVIS spectrum of the methanolic MnP solution. The Soret band appeared at 457 nm. The UVVIS spectra of the supernatant collected after the immobilization process (
Figure 8c) confirmed MnP immobilization close to 100% for all the prepared solids.
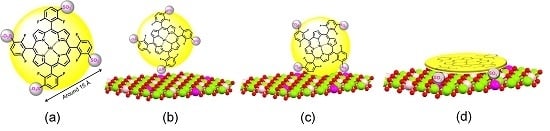
We excluded the interaction of the MnP with the layered crystal edges, which was negligible in terms of quantity. Then, we attempted to understand the mode of MnP immobilization on the surface of the LDH support by performing some calculations.
Considering the ideal M+2/M+3 molar ratios used in the synthesis, where the M+3 concentration led to a proportional concentration of positive charges on the surface of the layered crystals, we expected that the tetranegatively charged MnP would establish different modes of interaction.
Because both adjacent layers should share the charges, the positive charge on the layer surface should be half of the value and the distances between charges should be twice the distance between the M
+3 cations. This calculation was based on the values found for the cell “a” parameter (
Table 2), which measures the distance between the metal ions present in the layers (
Table 5). As for d
1,2, it represents the distance between the numbered purple balls labeled 1 and 2 in
Figure 9. The same representation applies for the distances between purple balls labeled 2 and 3 (d
2,3), 3 and 4 (d
3,4), 4 and 1 (d
4,1), 1 and 3 (d
1,3) and 2 and 4 (d
2,4).
Software
Hyperchem® helped to determine the distances between the MnP negative charges (SO
3−) (
Figure 10a) by optimization of the MnP structure.
Figure 10b–d show a schematic representation of the possible modes of MnP interaction with the layered crystal surface.
The first proposal (
Figure 10d) represents interaction of four negative charges in the MnP with four positive charges in the support layer. For the MnP to immobilize on the LDH in this way, the calculated distances between the positive charges present in the support layer must precisely match the distances between the four MnP negative charges. This limitation allowed us to exclude this arrangement from the formulated hypothesis.
In the second representation (
Figure 10c), two MnP negative charges interact with LDH. Values described in
Table 5 were based on the distance between two LDH positive charges and on the distance between the MnP SO
3− substituents (negative charges). Analysis of the values of positive charges in the LDH layers presented in
Table 5 (indicated by *) suggested that this kind of interaction (by two charges) was possible for MnP immobilization on the LDH prepared at only M
2+/M
3+ molar ratio of 4:1.
Figure 10b corresponds to yet another proposal for MnP immobilization on the LDH-MnP would be in contact with the LDH surface just via one negative charge. This is probably the most feasible proposal because it only depends on the approach of one opposite charge and dismisses the need to match the distances between the MnP negative charges and the LDH surface layer positive charges.
We evaluated the prepared solids as catalysts for cyclooctene oxidation in an attempt to understand how the M
+2/M
+3 molar ratio, the type of intercalated anion (single-charged nitrate and double-charged carbonate), and the mode of MnP immobilization influenced the MnP catalytic activity. Cyclooctene was selected because it is commonly employed as substrate during investigation into the catalytic activity of MPs [
16,
17,
83,
100,
101].
Although all the solid catalysts contained the same MnP, they afforded distinct catalytic results (
Table 6). Based on
Table 6, rationalizing the effect of the support on product yield was not easy. However, analysis of the last column of
Table 6, which provided the epoxide yields by excluding the yield obtained for the control reactions (reactions performed with pure LDH without immobilized MnP,
Table 6, runs 13–23) revealed some interesting trends in the catalytic behavior of the MnP immobilized on the prepared LDHs.
Figure 11 represents these differences and trends better.
MnP immobilization on LDH5, LDH10, LDH11, and LDH2 (red bars in
Figure 11) produced solid catalysts that gave higher product yields than the same MnP during homogeneous catalysis (magenta bar in
Figure 11). Most of these prepared LDHs contained high Zn/Al molar ratio. The intercalated anion did not seem to affect the catalytic results. The only exception to this trend was LDH2, which corresponded to a Mg/Al-LDH but had the same M
2+/M
3+ molar ratio as LDH5 (3:1). Indeed, the catalytic result achieved with MnP-LDH2 was on the borderline between the red and the green group in
Figure 11. All the solids in the red group gave rise to higher catalytic performance as compared to the MnP in homogeneous solution.
The group of green bars in
Figure 11 represented the solid catalysts that gave product yield similar to the yield obtained with the same MnP during homogeneous catalysis, namely MnP-LDH9 (Zn/Al 2:1), MnP LDH4 (Zn/Al 2:1), and, at the borderline between the green and blue groups, MnP-LDH6 (Mg/Al 2:1). The catalysts in the green group showed that MnP immobilization did not translate into the catalytic advantage observed in the case of the catalysts in the red group. The only advantage of MnP immobilization in the green group was prevention of MnP catalytic activity loss and possibility of catalyst recovery and reuse after the first cycle.
Finally, the blue group corresponded to the solid catalysts that afforded lower epoxidation yield than the yield furnished by the MnP during homogeneous catalysis, more specifically MnP-LDH7 (Mg/Al 3:1), MnP-LDH1 (Mg/Al 2:1), MnP-LDH3 (Mg/Al 4:1), and MnP-LDH8 (Mg/Al 4:1). All the LDHs in this group contained Mg2+ in their compositions, showing that this ion abated the MnP catalytic performance irrespective of the intercalated anion and of the M2+/M3+ molar ratio.
These results suggested that the characteristics of the MnP/support catalytic system depended on the LDH and on the kind of interaction established between the MnP and the support. At a first glance, one might think that the access of reagents to the MnP was a function of the divalent metal present in the LDH.
M2+/M3+ molar ratios of 3:1 in the LDH seemed to favor the MnP catalytic activity. This may have been related to the way that the porphyrin attached to the LDH surface and showed that changes in support composition may have affected the approach of the substrate to the catalytically active center. This effect was more evident for Zn/Al-LDH.
In general, LDH containing Zn2+ ions furnished the best catalytic results. The effect of zinc versus magnesium is not easy to explain, and studies on how different LDH compositions impact the catalytic oxidation results are rare.
Zhang
et al. recently prepared gold nanoclusters (AuNCs) supported on M
3Al-LDHs (M = Mg
2+, Ni
2+, Co
2+) and used these solids to catalyze aerobic 1-phenylethanol oxidation under solvent-free conditions [
102]. The AuNCs/M
3Al-LDH (where M = Ni
2+ or Co
2+) catalysts presented higher alcohol oxidation activity than AuNCs/Mg
3Al-LDH. Many factors contributed to these results: (i) generation of a larger amount of hydroxyl groups in the presence of Ni
2+ and Co
2+ during preparation of the M
3Al-LDH support, which implied that Brønsted basic sites existed in the AuNCs/M
3Al-LDH catalysts; (ii) high acidity of the LDH surface in the presence of AuNCs; (iii) critical effect of the metal components in the LDH supports on the electronic structure of AuNCs; (iv) more negatively charged AuNCs in AuNCs/Ni
3Al-LDH and AuNCs/Co
3Al-LDH; (v) larger electronegativity of the transition metals Ni and Co as compared to Mg, which may have facilitated electron transfer from the support to the AuNCs, resulting in more electron-rich Au cores in AuNCs/Ni
3Al-LDH and AuNCs/Co
3Al-LDH than in AuNCs/Mg
3Al-LDH. The authors claimed that all these factors elicited a synergistic effect between the LDH and AuNCs, resulting in better catalytic performance for AuNCs/Ni
3Al-LDH and AuNCs/Co
3Al-LDH during the oxidation reaction.
In another recent example, Sun
et al. [
103] prepared a structured catalyst by immobilizing a cobalt phthalocyanine tetrasulfonate (CoPcS) onto multimetallic oxides of the Mg,Ni/Al-MMO type (obtained by thermal decomposition of Mg,Ni/Al-LDH) with tunable basicity. Their purpose was to enhance the synergistic effect between the active center (CoPcS) and the basicity of the support to the maximal extent; they used the Mercaptan Sweetening catalytic reaction via oxidation of 1-octanethiol to the corresponding disulfide as model reaction [
101]. Changes in the relative content of Mg
2+ and Ni
2+ in the LDH precursor used to prepare the support Mg,Ni/Al-MMO by thermal decomposition conveniently tuned the basicity and the number of moderately basic sites in the solid designated CoPcS/Mg,Ni/Al-MMO. Alterations in the Mg/Ni molar ratios increased the conversion value: Mg/Ni molar ratio of 1.16 gave maximum conversion (93%). The authors claimed that the synergistic effect between the oxidation center (CoPcS) and the large number of moderate basic sites (0.49 mmol/g, determined by CO
2-TPD) present in the catalyst support was optimal at this Mg/Ni molar ratio. Thereafter, conversion values decreased. The authors concluded that the intrinsic mechanism of the synergistic should be rather complicated, which is the reason why it is under investigation in their research lab.
Together, these few examples corroborated our preliminary results on the active influence of the catalyst support on the oxidative catalytic activity of the MnP during cyclooctene oxidation.
The possible effect of the water present in the interlayer space and outer surface of the prepared LDHs should not be ignored. Percentage H
2O mass loss in the solid LDHs without MnP (
Table 3) and the cyclooctene oxidation reaction yields achieved for the respective solids (
Table 6) pointed to a dependence of product yield on the quantity of water in the solid. In other words, water might affect the catalytic behavior of the material in these reactions [
103].
In conclusion, the catalytic results presented here suggested that the catalytic properties of the investigated MnP-LDH solids strongly depended on the nature of the LDH support, the M+2/M+3 molar ratio, and the intercalated anion, which consequently influenced the mode of negatively charged MnP immobilization on the layered crystals surface.
4. Conclusions
Layered compounds are a very interesting class of compounds for the immobilization of porphyrins, metalloporphyrins (MP), phthalocyanines, and other macrocyclic complexes. Immobilization may occur on the layered surface or between the layers (intercalation).
Layered double hydroxides (LDHs) have long been investigated as supports for MP immobilization. In recent years, new catalysts have been developed by diverse strategies like LDH exfoliation, LDH restacking, low-polar MP encapsulation into LDH layers intercalated with dodecyl sulfonate, and MP entrapment into LDHs anchored on magnetic particles. The latter alternative facilitates catalyst recovery and reuse because a simple magnet can separate the catalyst from the reaction medium.
Some layered hydroxide salts (LHSs) such as zinc hydroxide nitrate (ZHN) and zinc hydroxide chloride (ZHC) display distinct behavior regarding MP immobilization, including modified selectivity of the supported catalyst during cyclohexane oxidation, which indicates that the support influences the oxidation mechanism. In addition, zinc oxides derived from LHSs exert cooperative effects on immobilized MP during catalytic toluene oxidation.
MPs immobilized on LHSs remain unexplored. Many LHSs can be used for investigative purposes. LHSs may contain Cu2+, Co2+, Ni2+, Mn2+, and mixtures of two or even three different divalent metals as well as intercalated hydrophyllic anions (such as NO3−, Cl−, SO42−, and CO32−) or hydrophobic interlayer anions (like carboxylates, sulfonates, and phosphates). In the case of organic divalent anionic species bearing one negative charge at the end of the ion, grafting of the species to the support is also possible, and pillared derivatives with high surface area may arise. If by only changing the interlayer anion in the support bearing the anionic FeP (e.g., ZHN and ZHC) greatly modifies the oxidation mechanism, more surprises may lie ahead for MP immobilization on LHSs. Moreover, MP immobilization on LHS oxide derivatives can aid the development of novel catalysts for oxidation or photocatalytic reactions.
This work also reported on immobilization of a manganese(III) porphyrin [Mn(TDFSPP)] (MnP) on different LDHs prepared with variable M+2/M+3 molar ratios (M+2 = Mg+2 or Zn+2 and M+3 = Al+3) and different intercalated anions (monovalent nitrate and divalent carbonate) to investigate how these variables affected MnP immobilization and catalytic performance. Preliminary catalytic results suggested that composition of the prepared LDHs seemed to impact the catalytic performance of the immobilized MnP. Distinct divalent metals (Mg+2 or Zn+2) in the LDH elicited different results. The M2+/M3+ molar ratio in the support correlated positively with the catalytic activity. Probably, changes in the basicity of the support and not the spacing between the positive charges in the layers (i.e., the MP mode of immobilization) influenced the MnP catalytic activity. This proposition is currently under investigation in our research lab.