A Peptoid-Based Fluorescent Sensor for Cyanide Detection
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Coumarin-Attached Peptoids
2.2. Spectroscopic Features of Free CP3
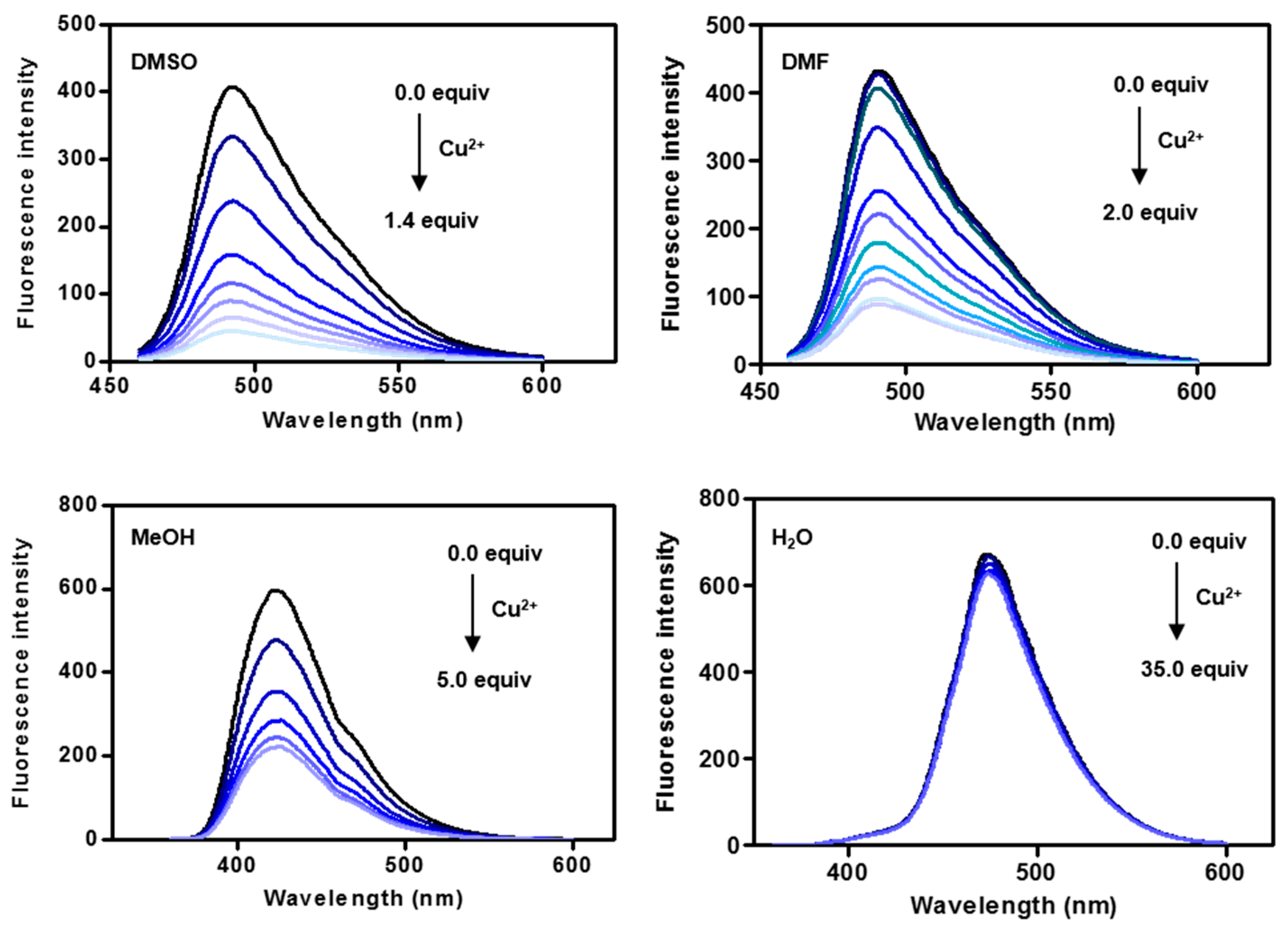
2.3. Fluorescence Changes with Formation of the CP3-Cu2+ Complex
2.4. The CP3-Cu2+ Complex as a Cyanide Sensor
2.5. CP4 Displays Different Spectroscopic Features to CP3
3. Discussion
4. Experimental Section
4.1. General
4.2. Synthesis of N-(2-Amino-2-oxoethyl)-2-(2-(benzylamino)-N-(2-methoxyethyl)acetamido)-N-(prop-2-yn-1-yl)acetamide (Solid Phase Peptide Synthesis) (1)
4.3. Synthesis of 3-Azido-7-hydroxycoumarin (2a) and 3-Azido-7-methoxycoumarin (2b)
4.4. Synthesis of N-(2-Amino-2-oxoethyl)-2-(2-(benzylamino)-N-(2-methoxyethyl)acetamido)-N-((1-(7-hydroxy-2-oxo-2h-chromen-3-yl)-1h-1,2,3-triazol-4-yl)methyl)acetamide (CP3)
4.5. Synthesis of N-(2-Amino-2-oxoethyl)-2-(2-(benzylamino)-N-(2-methoxyethyl)acetamido)-N-((1-(7-methoxy-2-oxo-2h-chromen-3-yl)-1h-1,2,3-triazol-4-yl)methyl)acetamide (CP4)
4.6. Spectroscopic Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Figliozzi, G.M.; Goldsmith, R.; Ng, S.C.; Banville, S.C.; Zuckermann, R.N. Synthesis of n-substituted glycine peptoid libraries. Methods Enzymol. 1996, 267, 437–447. [Google Scholar] [PubMed]
- Culf, A.S.; Ouellette, R.J. Solid-phase synthesis of n-substituted glycine oligomers (α-peptoids) and derivatives. Molecules 2010, 15, 5282–5335. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, L.; Orzaez, M.; Sanclimens, G.; Moure, A.; Arminan, A.; Sepulveda, P.; Messeguer, A.; Vicent, M.J.; Perez-Paya, E. Modulation of cellular apoptosis with apoptotic protease-activating factor 1 (α-1) inhibitors. J. Med. Chem. 2008, 51, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Udugamasooriya, D.G.; Dineen, S.P.; Brekken, R.A.; Kodadek, T. A peptoid “antibody surrogate” that antagonizes vegf receptor 2 activity. J. Am. Chem. Soc. 2008, 130, 5744–5752. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Martin, E.J.; Spellmeyer, D.C.; Stauber, G.B.; Shoemaker, K.R.; Kerr, J.M.; Figliozzi, G.M.; Goff, D.A.; Siani, M.A.; Simon, R.J. Discovery of nanomolar ligands for 7-transmembrane g-protein-coupled receptors from a diverse n-(substituted)glycine peptoid library. J. Med. Chem. 1994, 37, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Durell, S.R.; Myers, M.C.; Appella, D.H. Probing the structural requirements of peptoids that inhibit HDM2-p53 interactions. J. Am. Chem. Soc. 2006, 128, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.M.; Zuckermann, R.N.; Reinhard, C.; Frey, W.H., II. Intranasal administration delivers peptoids to the rat central nervous system. Neurosci. Lett. 2008, 439, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Metallopeptoids. Chem. Commun. 2009, 7, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.A.; Blackwell, H.E. Structure-function relationships in peptoids: Recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 2009, 7, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.M.; Jang, H.; Kirshenbaum, K. Clickity-click: Highly functionalized peptoid oligomers generated by sequential conjugation reactions on solid-phase support. Org. Biomol. Chem. 2006, 4, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.S.; Engel-Andreasen, J.; Fristrup, P.; Harris, P.; Olsen, C.A. Cis-trans amide bond rotamers in β-peptoids and peptoids: Evaluation of stereoelectronic effects in backbone and side chains. J. Am. Chem. Soc. 2013, 135, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Sanclimens, G.; Bujons, J.; Masip, I.; Alvarez-Larena, A.; Perez-Paya, E.; Alfonso, I.; Messeguer, A. Chemical modulation of peptoids: Synthesis and conformational studies on partially constrained derivatives. Chemistry 2011, 17, 7927–7939. [Google Scholar] [CrossRef] [PubMed]
- Caumes, C.; Roy, O.; Faure, S.; Taillefumier, C. The click triazolium peptoid side chain: A strong cis-amide inducer enabling chemical diversity. J. Am. Chem. Soc. 2012, 134, 9553–9556. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.A.; Seidl, F.J.; Bruno, P.A.; Plescia, M.A.; Palla, K.S. Use of the environmentally sensitive fluorophore 4-N,N-dimethylamino-1,8-naphthalimide to study peptoid helix structures. Biopolymers 2011, 96, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Clark, R.J.; Zhu, L. Highly sensitive fluorescent probes for zinc ion based on triazolyl-containing tetradentate coordination motifs. Org. Lett. 2007, 9, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.; Lippard, S.J. Direct detection of nitroxyl in aqueous solution using a tripodal copper(II) bodipy complex. J. Am. Chem. Soc. 2010, 132, 5536–5537. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, E.A.; Gantzel, P.; Benedetti, E.; Goodman, M. A multinuclear Ca2+ complex of a linear n-protected glycyl-dipeptoid derivative. J. Am. Chem. Soc. 1997, 119, 3187–3188. [Google Scholar] [CrossRef]
- Lee, B.C.; Chu, T.K.; Dill, K.A.; Zuckermann, R.N. Biomimetic nanostructures: Creating a high-affinity zinc-binding site in a folded nonbiological polymer. J. Am. Chem. Soc. 2008, 130, 8847–8855. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G. Conformational control in metallofoldamers: Design, synthesis and structural properties. Eur. J. Org. Chem. 2009, 2009, 5699–5710. [Google Scholar] [CrossRef]
- Izzo, I.; Ianniello, G.; De Cola, C.; Nardone, B.; Erra, L.; Vaughan, G.; Tedesco, C.; De Riccardis, F. Structural effects of proline substitution and metal binding on hexameric cyclic peptoids. Org. Lett. 2013, 15, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Zhou, E.Y.; Pelton, J.G.; Francis, M.B. Selective chromium(VI) ligands identified using combinatorial peptoid libraries. J. Am. Chem. Soc. 2013, 135, 17488–17493. [Google Scholar] [CrossRef] [PubMed]
- Maayan, G.; Zabrodski, T.; Baskin, M.; Kaniraj, P. Click to bind: Microwave-assisted solid-phase synthesis of peptoids incorporating pyridine–triazole ligands and their copper(II) complexes. Synlett 2014, 26, 461–466. [Google Scholar] [CrossRef]
- Nalband, D.M.; Warner, B.P.; Zahler, N.H.; Kirshenbaum, K. Rapid identification of metal-binding peptoid oligomers by on-resin x-ray fluorescence screening. Biopolymers 2014, 102, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B. Development of peptoid-based ligands for the removal of cadmium from biological media. Chem. Sci. 2015, 6, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Prathap, K.J.; Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 2015, 51, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Baskin, M.; Maayan, G. A rationally designed metal-binding helical peptoid for selective recognition processes. Chem. Sci. 2016. [Google Scholar] [CrossRef]
- De Ricco, R.; Potocki, S.; Kozlowski, H.; Valensin, D. Nmr investigations of metal interactions with unstructured soluble protein domains. Coord. Chem. Rev. 2014, 269, 1–12. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, J.H.; Habata, Y.; Kang, C.; Kim, J.S. An iminocoumarin-cu(II) ensemble-based chemodosimeter toward thiols. Chem. Commun. 2011, 47, 5142–5144. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, X.; Pan, J.; Spring, D.R. Coumarin-derived transformable fluorescent sensor for Zn2+. Chem. Commun. 2012, 48, 4764–4766. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Li, K.; Yu, K.K.; Wu, M.Y.; Yu, X.Q. Coumarin-dpa-cu(II) as a chemosensing ensemble towards histidine determination in urine and serum. Org. Biomol. Chem. 2013, 11, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-T.; Chen, W.-C.; Liu, S.-R.; Wu, S.-P. A coumarin-based sensitive and selective fluorescent sensor for copper(II) ions. New J. Chem. 2014, 38, 4434–4439. [Google Scholar] [CrossRef]
- Kim, M.J.; Swamy, K.M.; Lee, K.M.; Jagdale, A.R.; Kim, Y.; Kim, S.J.; Yoo, K.H.; Yoon, J. Pyrophosphate selective fluorescent chemosensors based on coumarin-DPA-Cu(II) complexes. Chem. Commun. 2009, 7215–7217. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, O.; Cassels, B.K.; Perez, C.; Mena, N.; Nunez, M.T.; Martinez, N.P.; Pavez, P.; Aliaga, M.E. Coumarin-based fluorescent probes for dual recognition of copper(II) and iron(III) ions and their application in bio-imaging. Sensors 2014, 14, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Gueogjan, K.; Manarin, F.; Farsky, S.H.; Zukerman-Schpector, J.; Caracelli, I.; Pizano Rodrigues, S.R.; Muscara, M.N.; Teixeira, S.A.; Santin, J.R.; et al. Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: Effect on inducible nitric oxide synthase. Eur. J. Med. Chem. 2012, 58, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.Y.; Luo, H.; Liu, H.; Han, X.; Yan, G.Y.; Ding, Z.Y.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.A.; Zhou, C.H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.T.; Lai, T.L.; Wu, R.T.; Tsai, M.T.; Wu, C.M.; Lee, G.H.; Chung, W.S. Design and synthesis of triazolyl coumarins as Hg2+ selective fluorescent chemosensors. Analyst 2012, 137, 5770–5776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, K.; Li, J.-Y.; Fang, Y.; Zhao, T.-C.; Yao, C. Visualization of nitroxyl in living cells by a chelated copper(II) coumarin complex. Org. Lett. 2011, 13, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.T.; Wei, X.L.; Sheng, Y.; Zang, Y.; He, X.P.; Xie, J.; Liu, G.; Tang, Y.; Li, J.; Chen, G.R. Substitution pattern reverses the fluorescence response of coumarin glycoligands upon coordination with silver(I). Sci. Rep. 2014, 4, 4252. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Govindaraju, T. Conformationally constrained (coumarin-triazolyl-bipyridyl) click fluoroionophore as a selective Al3+ sensor. Inorg. Chem. 2010, 49, 7229–7231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Han, J.H.; Kim, Z.H.; Kang, C.; Kim, J.S. Coumarin-cu(II) ensemble-based cyanide sensing chemodosimeter. Org. Lett. 2011, 13, 5056–5059. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, J.; Xiong, W.W.; Li, P.Z.; Zhang, H.; Zhao, Y.; Zhang, Q. Pyridinium-fused pyridinone: A novel “turn-on” fluorescent chemodosimeter for cyanide. Chem. Asian J. 2014, 9, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.-J.; Guo, Y.; Yang, X.-F.; Suzenet, F.; Li, J.; Li, C.-W.; Duan, Y.-W. Coumarin–hemicyanine conjugates as novel reaction-based sensors for cyanide detection: Convenient synthesis and ict mechanism. RSC Adv. 2014, 4, 19077–19085. [Google Scholar] [CrossRef]
- Fisher, A.E.O.; Naughton, D.P. Novel peptoids for the detection and suppression of reactive oxygen and nitrogen species. Biochem. Soc. Trans. 2003, 31, 1302–1304. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.A.; Holmes, C.A.; Seidl, F.J. A fluorescent peptoid ph-sensor. Biopolymers 2013, 100, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Burkoth, T.S.; Fafarman, A.T.; Charych, D.H.; Connolly, M.D.; Zuckermann, R.N. Incorporation of unprotected heterocyclic side chains into peptoid oligomers via solid-phase submonomer synthesis. J. Am. Chem. Soc. 2003, 125, 8841–8845. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, K.; Xie, F.; Cash, B.M.; Long, S.; Barnhill, H.N.; Wang, Q. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarins and acetylenes. Org. Lett. 2004, 6, 4603–4606. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Henry, E.; Mani, N.K.; Tang, J.; Brochon, J.-C.; Deprez, E.; Xie, J. Click chemistry to fluorescent amino esters: Synthesis and spectroscopic studies. Eur. J. Org. Chem. 2010, 2010, 2395–2405. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Berberan-Santos, M.N. Chemical sensing via fluorescence. In Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 409–478. [Google Scholar]
- Kurnia, K.; Giles, D.E.; May, P.M.; Singh, P.; Hefter, G.T. Cyanide thermodynamics 2. Stability constants of copper(I) cyanide complexes in aqueous acetonitrile mixtures. Talanta 1996, 43, 2045–2051. [Google Scholar] [CrossRef]
- Westlake, B.C.; Paul, J.J.; Bettis, S.E.; Hampton, S.D.; Mehl, B.P.; Meyer, T.J.; Papanikolas, J.M. Base-induced phototautomerization in 7-hydroxy-4-(trifluoromethyl)coumarin. J. Phys. Chem. B 2012, 116, 14886–14891. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Katano, K.; Hashimoto, T.; Hayashita, T. Solvent effect on the fluorescence response of hydroxycoumarin bearing a dipicolylamine binding site to metal ions. Anal. Sci. 2014, 30, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, I.; Trendafilova, N.; Aquino, A.J.A.; Lischka, H. Excited-state proton transfer in 7-hydroxy-4-methylcoumarin along a hydrogen-bonded water wire. J. Phys. Chem. A 2007, 111, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Moriay, T. Excited-state reactions of coumarins in aqueous solutions. I. The phototautomerization of 7-hydroxycoumarin and its derivative. Bull. Chem. Soc. Jpn. 1983, 56, 6–14. [Google Scholar] [CrossRef]
- Shang, L.; Zhang, L.H.; Dong, S.J. Turn-on fluorescent cyanide sensor based on copper ion-modified cdte quantum dots. Analyst 2009, 134, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, J.; Liu, X.; Wang, H. A highly selective pyrene based “off–on” fluorescent chemosensor for cyanide. New J. Chem. 2013, 37, 3869–3872. [Google Scholar] [CrossRef]
- Tanaka, T.; Mizuno, T.; Fukui, S.; Hiroaki, H.; Oku, J.; Kanaori, K.; Tajima, K.; Shirakawa, M. Two-metal ion, Ni(II) and Cu(II), binding alpha-helical coiled coil peptide. J. Am. Chem. Soc. 2004, 126, 14023–14028. [Google Scholar] [CrossRef] [PubMed]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Nmr studies on Cu(II)-peptide complexes: Exchange kinetics and determination of structures in solution. Mol. BioSyst. 2005, 1, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not avaiable.







| Wavelength | CHCl3 | MeOH | DMF | DMSO | H2O |
|---|---|---|---|---|---|
| λex (nm) | 357 | 347 | 347/441 | 347/441 | 344 |
| λem (nm) | 425 | 423 | 429/492 | 429/492 | 475 |
| Stokes shift (nm) | 68 | 76 | 82/51 | 82/51 | 131 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.; Lee, J. A Peptoid-Based Fluorescent Sensor for Cyanide Detection. Molecules 2016, 21, 339. https://doi.org/10.3390/molecules21030339
Lim B, Lee J. A Peptoid-Based Fluorescent Sensor for Cyanide Detection. Molecules. 2016; 21(3):339. https://doi.org/10.3390/molecules21030339
Chicago/Turabian StyleLim, Bumhee, and Jeeyeon Lee. 2016. "A Peptoid-Based Fluorescent Sensor for Cyanide Detection" Molecules 21, no. 3: 339. https://doi.org/10.3390/molecules21030339
APA StyleLim, B., & Lee, J. (2016). A Peptoid-Based Fluorescent Sensor for Cyanide Detection. Molecules, 21(3), 339. https://doi.org/10.3390/molecules21030339





