On the High Sensitivity of the Electronic States of 1 nm Gold Particles to Pretreatments and Modifiers
Abstract
:1. Introduction
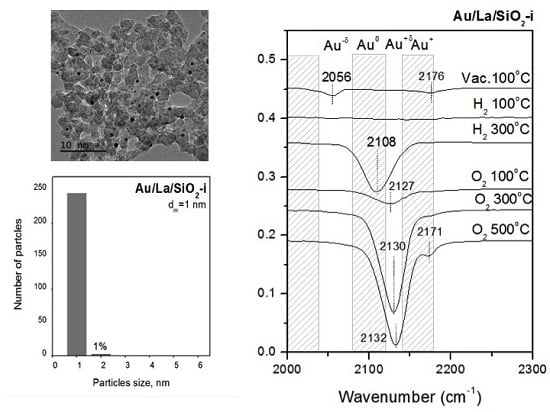
2. Results and Discussion
3. Materials and Methods
3.1. Support Preparation
3.2. Catalysts Preparation
3.3. High Resolution Transmission Electronic Microscopy
3.4. FTIR Spectra of Adsorbed CO
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ac-HAADF/STEM | aberration-corrected annual dark field scanning transmission microscope |
| Au NPs | gold nanoparticles |
| HRTEM | high resolution transmission electron microscopy |
| WGS | water-gas shift reaction |
| TPR | temperature-programmed reduction |
| FTIR CO | Fourier transform infrared spectroscopy of adsorbed CO |
| MSI | metal-support interaction |
References
- Laguna, O.H.; Romero Sarria, F.; Centeno, M.A.; Odriozola, J.A. Gold supported on metal-doped ceria catalysts (M = Zr, Zn and Fe) for the preferential oxidation of CO (PROX). J. Catal. 2010, 276, 360–370. [Google Scholar] [CrossRef]
- Gonzalez Castaño, M.; Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Pt vs. Au in water–gas shift reaction. J. Catal. 2014, 314, 1–9. [Google Scholar] [CrossRef]
- Tuzovskaya, I.; Lima, E.; Bosch, P.; Bogdanchikova, N.; Pestryakov, A.; Fraissard, J. Influence of cation nature on stabilization of gold nanospecies in mordenites. J. Nanosci. Nanotechnol. 2011, 11, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Riahi, G.; Guillemot, D.; Polisset-Thfoin, M.; Khodadadi, A.A.; Fraissard, J. Preparation, characterization and catalytic activity of gold-based nanoparticles on HY zeolites. Catal. Today 2002, 72, 115–121. [Google Scholar] [CrossRef]
- Arrii, S.; Morfin, F.; Renouprez, A.J.; Rousset, J.L. Oxidation of CO on Gold supported catalysts prepared by laser vaporization: Direct evidence of support contribution. J. Am. Chem. Soc. 2004, 126, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Supported gold catalysts prepared by in situ reduction technique: Preparation, characterization and catalytic activity measurements. Appl. Catal. A Gen. 2004, 259, 163–168. [Google Scholar] [CrossRef]
- Yuan, Y.; Kozlova, A.P.; Asakura, K.; Wan, H.; Tsai, K.; Iwasawa, Y. Supported Au catalysts prepared from Au phosphine complexes and as-precipitated metal hydroxides: Characterization and low-temperature CO oxidation. J. Catal. 1997, 170, 191–199. [Google Scholar] [CrossRef]
- Kozlov, A.I.; Kozlova, A.P.; Asakura, K.; Matsui, Y.; Kogure, T.; Shido, T.; Iwasawa, Y. Supported gold catalysts prepared from a gold phosphine precursor and as-precipitated metal-hydroxide precursors: Effect of preparation conditions on the catalytic performance. J. Catal. 2000, 196, 56–65. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Kiener, C.; Wögerbauer, C.; Baiker, A. Preparation of supported gold catalysts for low-temperature CO oxidation via “size-controlled” gold colloids. J. Catal. 1999, 181, 223–232. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, M.; Stoltze, P.; Nørskov, J.K. Making gold less noble. Catal. Lett. 2000, 64, 101–106. [Google Scholar] [CrossRef]
- Hodge, N.A.; Kiely, C.J.; Whyman, R.; Siddiqui, M.R.H.; Hutchings, G.J.; Pankhurst, Q.A.; Wagner, F.E.; Rajaram, R.R.; Golunski, S.E. Microstructural comparison of calcined and uncalcined gold/iron-oxide catalysts for low-temperature CO oxidation. Catal. Today 2002, 72, 133–144. [Google Scholar] [CrossRef]
- Guzman, J.; Gates, B.C. Simultaneous presence of cationic and reduced gold in functioning MgO-supported CO oxidation catalysts: Evidence from X-ray absorption spectroscopy. J. Phys. Chem. 2002, 106, 7659–7665. [Google Scholar] [CrossRef]
- Edwards, J.K.; Solsona, B.E.; Landon, P.; Carley, A.F.; Herzing, A.; Kiely, C.J.; Hutchings, G.J. Direct synthesis of hydrogen peroxide from H2 and O2 using TiO2-supported Au-Pd catalysts. J. Catal. 2005, 236, 69–79. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.C.; Thompson, D.T. Gold-catalysed oxidation of carbon monoxide. Gold Bull. 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Kimble, M.L.; Castleman, A.W., Jr.; Mitrić, R.; Bürgel, C.; Bonacic-Koutecky, V. Reactivity of Atomic Gold Anions toward oxygen and the oxidation of CO: Experiment and theory. J. Am. Chem. Soc. 2004, 126, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Simakov, A.; Tuzovskaya, I.; Pestryakov, A.; Bogdanchikova, N.; Gurin, V.; Avalos, M.; Farías, M.H. On the nature of active gold species in zeolites in CO oxidation. Appl. Catal. A Gral. 2007, 331, 121–128. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Bando, K.K.; Lu, J.; Haruta, M.; Fujitani, T.; Oyama, S.T. Transient technique for identification of true reaction intermediates: Hydroperoxide species in propylene epoxidation on gold/titanosilicate catalysts by X-ray absorption fine structure spectroscopy. J. Phys. Chem. C 2008, 112, 1115–1123. [Google Scholar] [CrossRef]
- Chowdhury, B.; Bravo-Suárez, J.J.; Mimura, N.; Lu, J.; Bando, K.K.; Tsubota, S.; Haruta, M. In situ UV-vis and EPR study on the formation of hydroperoxide species during direct gas phase propylene epoxidation over Au/Ti-SiO2 catalyst. J. Phys. Chem. B 2006, 110, 22995–22999. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.D.; Valsamakis, I.; Flytzani-Stephanopoulos, M. Novel Au/La2O3 and Au/La2O2SO4 catalysts for the water–gas shift reaction prepared via an anion adsorption method. Chem. Commun. 2012, 48, 4857–4859. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman Analysis of mode softening in nanoparticle CeO2δ and Au-CeO2δ during CO oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Flytzani-Stephanopoulos, M. Gold Atoms stabilized on various supports catalyze the water gas shift reaction. Acc. Chem. Res. 2014, 47, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically active Au-O(OH)x species stabilized by alkali ions on zeolites and mesoporous oxides. Science 2014, 364, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Tsukuda, T. Preparation of ~1 nm Gold clusters confined within mesoporous silica and microwave-assisted catalytic application for alcohol oxidation. J. Phys. Chem. C 2009, 113, 13457–13461. [Google Scholar] [CrossRef]
- Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 2014, 47, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, J.; Yang, S.; Han, B.; Sang, X.; Liu, C.; Ma, X.; Yang, G. Ultra-small gold nanoparticles immobilized on mesoporous silica/graphene oxide as highly active and stable heterogeneous catalysts. Chem. Commun. 2015, 51, 4398–4401. [Google Scholar] [CrossRef] [PubMed]
- Tabakova, T.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Tenchev, K.; Vindigni, F.; Ioannides, T. CO-free hydrogen production over Au/CeO2-Fe2O3 catalysts: Part 1. Impact of the support composition on the performance for the preferential CO oxidation reaction. Appl. Catal. B Environ. 2011, 101, 256–265. [Google Scholar] [CrossRef]
- Manzoli, M.; Chiorino, A.; Boccuzzi, F. FTIR study of nanosized gold on ZrO2 and TiO2. Surf. Sci. 2003, 532–535, 377–382. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M. FTIR study of the electronic effects of CO adsorbed on gold nanoparticles supported on titania. Surf. Sci. 2000, 454–456, 942–946. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Lu, P.; Akita, T.; Ichikawa, S.; Haruta, M. Au/TiO2 nanosized samples: A catalytic, TEM, and FTIR study of the effect of calcination temperature on the CO oxidation. J. Catal. 2001, 202, 256–267. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T. FTIR study of the low-temperature water-gas shift reaction on Au/Fe2O3 and Au/TiO2 catalysts. J. Catal. 1999, 188, 176–185. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T.; Ilieva, L.; Iadakiev, V. Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production. Catal. Today 2002, 75, 169–175. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Sobczak, J.W.; Idakiev, V.; Andreeva, D. Effect of synthesis procedure on the low-temperature WGS activity of Au/ceria catalysts. Appl. Catal. B. Environ. 2004, 49, 73–81. [Google Scholar] [CrossRef]
- Zaera, F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem. Soc. Rev. 2014, 43, 7624–7663. [Google Scholar] [CrossRef] [PubMed]
- Kantcheva, M.; Samarskaya, O.; Ilieva, L.; Pantaleo, G.; Venezia, A.M.; Andreeva, D. In situ FT-IR investigation of the reduction of NO with CO over Au/CeO2-Al2O3 catalyst in the presence and absence of H2. Appl. Catal. B Environ. 2009, 88, 113–126. [Google Scholar] [CrossRef]
- Martynyuk, O.; Kotolevich, Y.; Pestryakov, A.; Mota-Morales, J.D.; Bogdanchikova, N. Nanostructures constituted by unusually small silica nanoparticles modified with metal oxides as support for ultra-small gold nanoparticles. Colloids Surf. A 2015, 487, 9–16. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Gómez, S.; Pawelec, B.; Zepeda, T.A. CO oxidation on Au nanoparticles supported on wormhole HMS material: Effect of support modification with CeO2. Appl. Catal. B Environ. 2009, 89, 128–136. [Google Scholar] [CrossRef]
- Gruver, V.; Fripiat, J.J. Lewis acid sites surface aluminum and mordenites. An infrared study of CO chemisorption. J. Phys. Chem. 1994, 98, 8549–8554. [Google Scholar] [CrossRef]
- Reifsnyder, S.N.; Otten, M.M.; Lamb, H.H. Nucleation and growth of Pd clusters in mordenite. Catal. Today 1998, 39, 317–328. [Google Scholar] [CrossRef]
- Salama, T.M.; Shido, T.; Minagawa, H.; Ichikawa, M. Characterization of Gold(I) in NaY Zeolite and Acidity Generation. J. Catal. 1995, 152, 322–330. [Google Scholar] [CrossRef]
- Guillemot, D.; Borovkov, V.Y.; Kazansky, V.B.; Polisset-Thfoin, M.; Fraissard, J. Surface characterization of Au/HY by 129Xe NMR and diffuse reflectance IR spectroscopy of adsorbed CO. Formation of electron-deficient gold particles inside HY cavities. J. Chem. Soc. Faraday Trans. 1997, 93, 3587–3591. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Mekkawy, I. Electrical and chemical characteristics of nano-meter gold encapsulated in mesoporous and microporous channels and cages of FSM-16 and Y zeolites. J. Phys. Chem. Solids 2003, 64, 299–306. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Andreeva, D. FTIR study of low-temperature water-gas shift reaction on gold/ceria catalyst. Appl. Catal. A Gen. 2003, 252, 385–397. [Google Scholar] [CrossRef]
- Margitfalvi, J.L.; Fási, A.; Hegedus, M.; Lónyi, F.; Gobölös, S.; Bogdanchikova, N. Au/MgO catalysts modified with ascorbic acid for low temperature CO oxidation. Catal. Today 2002, 72, 157–169. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Kharlanov, A.N.; Kochubey, D.I.; Bogdanchikova, N.; Stakheev, A.Y. Influence of modifying additives on electronic state of supported gold. J. Mol. Struct. 2002, 642, 129–136. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Kharlanov, A.N.; Bogdanchikova, N.E.; Tuzovskaya, I.V. Electronic state of gold in supported clusters. Eur. Phys. J. D 2003, 24, 307–309. [Google Scholar] [CrossRef]
- Pestryakov, A.; Tuzovskaya, I.; Smolentseva, E.; Bogdanchikova, N.; Jentoft, F.C.; Knop-Gericke, A. Formation of gold nanoparticles in zeolites. Int. J. Mod. Phys. B 2005, 19, 2321–2326. [Google Scholar] [CrossRef]
- Tuzovskaya, I.V.; Simakov, A.V.; Pestryakov, A.N.; Bogdanchikova, N.E.; Gurin, V.V.; Farías, M.H.; Tiznado, H.J.; Avalos, M. Co-existance of various active gold species in Au-mordenite catalyst for CO oxidation. Catal. Commun. 2007, 8, 977–980. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Bogdanchikova, N.; Simakov, A.; Tuzovskaya, I.; Jentoft, F.; Farias, M.; Díaz, A. Catalytically active gold clusters and nanoparticles for CO oxidation. Surf. Sci. 2007, 601, 3792–3795. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Tuzovskaya, I.; Zepeda, T.A.; Farias, M.H.; Tiznado, H.; Martynyuk, O. Effect of redox treatments on activation and deactivation of gold nanospecies supported on mesoporous silica in CO oxidation. Fuel 2013, 110, 40–47. [Google Scholar] [CrossRef]
- Martínez-González, S.; Gómez-Avilés, A.; Martynyuk, O.; Pestryakov, A.; Bogdanchikova, N.; Corberán, V.C. Selective oxidation of 1-octanol over gold supported on mesoporous metal-modified HMS: The effect of the support. Catal. Today 2014, 227, 65–70. [Google Scholar] [CrossRef]
- Pawelec, B.; Castaño, P.; Zepeda, T.A. Morphological investigation of nanostructured CoMo catalysts. Appl. Surf. Sci. 2008, 254, 4092–4102. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; Rombi, E.; Cannas, C.; Casu, M.; Virga, A.; Fiorilli, S.; Onida, B.; Ferino, I. Synthesis, characterization and catalytic activity of Au supported on functionalized SBA-15 for low temperature CO oxidation. J. Mater. Sci. 2009, 44, 6644–6653. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.-H.; Luo, Y.-C.; Mou, C.-Y.; Lin, S.D.; Cheng, H.; Chen, J.-M.; Lee, J.-F.; Lin, T.-S. Strong metal-support interactions between gold nanoparticles and ZnO nanorods in CO oxidation. J. Am. Chem. Soc. 2012, 134, 10251–10258. [Google Scholar] [CrossRef] [PubMed]
- Rombi, E.; Cutrufello, M.G.; Cannas, C.; Occhiuzzi, M.; Onida, B.; Ferino, I. Gold-assisted E′ centres formation on the silica surface of Au/SBA-15 catalysts for low temperature CO oxidation. Phys. Chem. Chem. Phys. 2012, 14, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Tanev, P.T.; Chibwe, M.; Pinnavaia, T.J. Titanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic compounds. Nature 1994, 368, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Tanev, P.T.; Pinnavaia, T.J. A neutral templating route to Mesoporous molecular sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Smolentseva, E.; Bogdanchikova, N.; Simakov, A.; Pestryakov, A.; Tusovskaya, I.; Avalos, M.; Farías, M.H.; Díaz, J.A.; Gurin, V. Influence of copper modifying additive on state of gold in zeolites. Surf. Sci. 2006, 600, 4256–4259. [Google Scholar] [CrossRef]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition-precipitation with NaOH and urea. Appl. Catal. A 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Zanella, R.; Louis, C. Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal. Today 2005, 107–108, 768–777. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all materials synthesized are available from the authors.





| Sample Content | Au wt %/M wt % | SBET, cm/m2 | |
|---|---|---|---|
| Support | Catalyst | ||
| Au/La/TiO2 | 3.6/5 | 45 | 45 |
| Au/Ce/TiO2 | 3.5/5 | 43 | 47 |
| Au/Mg/TiO2 | 4.0/1 | 43 | 44 |
| Au/Fe/TiO2 | 4.2/2 | 46 | 44 |
| Au/TiO2 | 4.5/0 | 56 | 46 |
| Au/La/SiO2-s | 2.3/7 | 325 | 110 |
| Au/La/SiO2-i | 2.4/7 | 242 | 145 |
| Au/FexOy/SiO2-s | 2.8/3 | 422 | 334 |
| Au/FexOy/SiO2-i | 2.5/3 | 459 | 195 |
| Au/CeO2/SiO2-s | 2.7/7 | 271 | 271 |
| Au/SiO2 | 2.5/0 | 345 | 228 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynyuk, O.; Kotolevich, Y.; Vélez, R.; Cabrera Ortega, J.E.; Tiznado, H.; Zepeda Partida, T.; Mota-Morales, J.D.; Pestryakov, A.; Bogdanchikova, N. On the High Sensitivity of the Electronic States of 1 nm Gold Particles to Pretreatments and Modifiers. Molecules 2016, 21, 432. https://doi.org/10.3390/molecules21040432
Martynyuk O, Kotolevich Y, Vélez R, Cabrera Ortega JE, Tiznado H, Zepeda Partida T, Mota-Morales JD, Pestryakov A, Bogdanchikova N. On the High Sensitivity of the Electronic States of 1 nm Gold Particles to Pretreatments and Modifiers. Molecules. 2016; 21(4):432. https://doi.org/10.3390/molecules21040432
Chicago/Turabian StyleMartynyuk, Oxana, Yulia Kotolevich, Rodrigo Vélez, Jesus Efren Cabrera Ortega, Hugo Tiznado, Trino Zepeda Partida, Josué D. Mota-Morales, Alexey Pestryakov, and Nina Bogdanchikova. 2016. "On the High Sensitivity of the Electronic States of 1 nm Gold Particles to Pretreatments and Modifiers" Molecules 21, no. 4: 432. https://doi.org/10.3390/molecules21040432