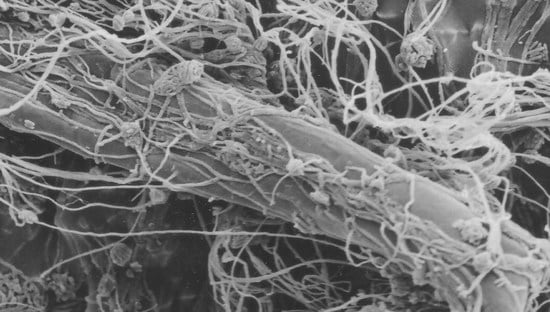
The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes
Abstract
:1. Introduction
2. Lecanicillium muscarium CCFEE 5003
3. Production and Characterization of Chitinolytic Enzymes by L. muscarium CCFEE 5003
4. Potential Applications of the Chitinolytic Enzymes by L. muscarium CCFEE 5003
5. Optimization of the Chitinolytic Enzymes Production in Bioreactors
6. Mycoparasitic Action of L. muscarium
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Vishniac, H.S. The microbiology of Antarctic soils. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 297–341. [Google Scholar]
- Onofri, S.; Pagano, S.; Zucconi, L.; Cicalini, A.R.; Selbmann, L.; Fenice, M. Microfungi in Antarctic extreme environments. Extracellular enzyme activities. In Proceedings of the IBC’s World Congress on Enzyme Technologies, San Francisco, CA, USA, 10–12 March 1999.
- Friedmann, E.I.; Thistle, A.B. Foreword. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. ix–x. [Google Scholar]
- Vincent, W.F.; Howard-Williams, C.; Broady, P.A. Microbial communities and processes in Antarctic flowing waters. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 544–569. [Google Scholar]
- Zucconi, L.; Pagano, S.; Fenice, M.; Selbmann, L.; Tosi, S.; Onofri, S. Growth temperature preferences of fungal strains from Victoria Land, Antarctica. Polar Biol. 1996, 16, 53–61. [Google Scholar] [CrossRef]
- Vincent, W.F. Microbial ecosystem of Antarctica; Cambridge University Press: Cambridge, UK, 1998; p. 304. [Google Scholar]
- Arcangeli, C.; Zucconi, L.; Onofri, S.; Cannistraro, S. Fluorescence study on whole Antarctic fungal spores under enhanced UV irradiation. J. Photoch. Photobiol. B 1997, 39, 258–264. [Google Scholar] [CrossRef]
- Vishniac, H.S. Psychrophilic yeasts. In Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Academic Publishers: Dordrecht, NL, USA, 1999; pp. 315–321. [Google Scholar]
- Azmi, O.R.; Seppelt, R.D. Fungi of the Windmill Islands, continental Antarctica. Effect of temperature, pH and culture media on the growth of selected microfungi. Polar Biol. 1997, 18, 128–134. [Google Scholar] [CrossRef]
- Barghini, P.; Moscatelli, D.; Garzillo, A.M.V.; Crognale, S.; Fenice, M. High production of cold-tolerant chitinases on shrimp wastes in bench-top bioreactor by the Antarctic fungus Lecanicillium muscarium CCFEE 5003: Bioprocess optimization and characterization of two main enzymes. Enzyme Microb. Technol. 2013, 53, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Felse, P.A.; Panda, T. Production of microbial chitinases. A revisit. Bioprocess. Eng. 2000, 23, 127–134. [Google Scholar] [CrossRef]
- Seidl, V. Chitinases of filamentous fungi: A large group of diverse proteins with multiple physiological functions. Fungal Biol. Rev. 2008, 22, 36–42. [Google Scholar] [CrossRef]
- Ulhoa, C.J.; Peberdy, J.F. Purification and some properties of extracellular chitinase produced by Trichoderma harzianum. Enzyme Microb. Tech. 1992, 14, 236–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, J.; Qiu, J.; Guan, X. Isolation and characterization of a chitinase gene from entomopathogenic fungus Verticillium lecanii. Braz. J. Microbiol. 2008, 39, 314–320. [Google Scholar]
- Rocha-Pino, Z.; Vigueras, G.; Shirai, K. Production and activities of chitinases and hydrophobins from Lecanicillium lecanii. Bioproc. Biosyst. Eng. 2011, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sandhya, C.; Suma, P.; Szakacs, G.; Pandey, A. Fungal biosynthesis of endochitinase and chitobiase in solid state fermentation and their application for the production of N-acetyl-d-glucosamine from colloidal chitin. Bioresour. Technol. 2007, 98, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Fenice, M.; Selbmann, L.; di Giambattista, R.; Petruccioli, M.; Federici, F. Production of extracellular chitinolytic activities by a strain of the Antarctic entomogenous fungus Verticillium cfr. lecanii. In Chitin Enzymology; Muzzarelli, R.A.A., Ed.; Atec Edizioni: Grottammare, Italy, 1996; pp. 285–292. [Google Scholar]
- Matsumoto, Y.; Saucedo-Castañeda, G.; Revah, S.; Shirai, K. Production of β-N-acetylhexosaminidase of Verticillium lecanii by solid state and submerged fermentations utilizing shrimp waste silage as substrate and inducer. Process. Biochem. 2004, 39, 665–671. [Google Scholar] [CrossRef]
- Fenice, M.; Gooday, G.W. Mycoparasitic actions against fungi and oomycetes by a strain (CCFEE 5003) of the fungus Lecanicillium muscarium isolated in Continental Antarctica. Ann. Microbiol. 2006, 56. [Google Scholar] [CrossRef]
- Ramírez-Coutiño, L.; Marín-Cervantes, M.; Huerta, S.; Revah, S.; Shirai, K. Enzymatic hydrolysis of chitin in the production of oligosaccharides using Lecanicillium fungicola chitinases. Process. Biochem. 2006, 41, 1106–1110. [Google Scholar] [CrossRef]
- Barghini, P.; Esti, M.; Pasqualetti, M.; Silvi, S.; Aquilanti, A.; Fenice, M. Crude cell wall degrading enzymes, by the Antarctic fungus Lecanicillium muscarium CCFEE 5003, inhibits the Ochratoxin-A producer Aspergillus carbonarius on white and red grapes. J. Environ. Prot. Ecol. 2013, 14, 1673–1679. [Google Scholar]
- Fenice, M.; di Giambattista, R.; Leuba, G.L.; Federici, F. Inactivation of Mucor plumbeus by the combined action of chitinase and high hydrostatic pressure. Int. J. Food Microbiol. 1999, 52, 109–113. [Google Scholar] [CrossRef]
- Malathrakis, N.E.; Kritsotaki, O. Effect of substrate, temperature and time of application on the effectiveness of three antagonistic fungi against Botrytis cinerea. In Recent Advances in Botrytis Research; Verhoeffer, K., Malathrakis, N.E., Williamson, B., Eds.; Pudoc Scientific Publisher: Wageningen, The Netherlands, 1992; pp. 187–191. [Google Scholar]
- Antal, Z.; Manczinger, L.; Szakacs, G.; Tengerdy, R.P.; Ferenczy, L. Colony growth, in vitro antagonism and secretion of extracellular enzymes in cold-tolerant strains of Trichoderma species. Mycol. Res. 2000, 104, 545–549. [Google Scholar] [CrossRef]
- Juarez-Jimenez, B.; Rodelas, B.; Martinez-Toledo, M.V.; Gonzalez-Lopez, J.; Crognale, S.; Gallo, A.M.; Pesciaroli, C.; Fenice, M. Production of chitinolytic enzymes by a strain (BM17) of Paenibacillus pabuli isolated from crab shells samples collected in the East Sector of Central Tyrrhenian Sea. Int. J. Biol. Macr. 2008, 43, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Gams, W. A revision of Verticillium sect. Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 2001, 73, 1–50. [Google Scholar]
- Askary, H.; Benhamou, N.; Brodeur, J. Ultrastructural and cytochemical characterization of aphid invasion by the hyphomycete Verticillium lecanii. J. Invertebr. Pathol. 1999, 74, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.K.; Lester, M.T.; Glare, T.R.; Christeller, J.T. The fungus, Lecanicillium muscarium, is an entomopathogen of passionvine hopper (Scolypopa australis). N. Z. J. Crop. Hort. 2003, 31, 1–7. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Walters, K.F.A.; Northing, P. The susceptibility of immature stages of Bemisia tabaci to the entomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 2005, 159, 23–29. [Google Scholar] [CrossRef] [PubMed]
- North, J.P.; Cuthbertson, A.G.; Walters, K.F. The efficacy of two entomopathogenic biocontrol agents against adult Thrips palmi (Thysanoptera: Thripidae). J. Invertebr. Pathol. 2006, 92, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Askary, H.; Yarmand, H. Development of the entomopathogenic hyphomycete Lecanicillium muscarium (Hyphomycetes: Moniliales) on various hosts. Eur. J. Entomol. 2007, 104, 67–72. [Google Scholar] [CrossRef]
- Goettel, M.S.; Koike, M.; Kim, J.J.; Aiuchi, D.; Shinya, R.; Brodeur, J. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. J. Invertebr. Pathol. 2008, 98, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Tiwary, B.N. Pathogenicity of entomopathogenic fungi to eggs and larvae of Spodoptera litura, the common cutworm. Biocontrol. Sci. Technol. 2009, 19, 919–929. [Google Scholar] [CrossRef]
- Anand, R.; Prasad, B.; Tiwary, B.N. Relative susceptibility of Spodoptera litura pupae to selected entomopathogenic fungi. BioControl 2009, 54, 85–92. [Google Scholar] [CrossRef]
- De Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. BiolControl 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Meyer, S.L.; Huettel, R.N.; Sayre, R.M. Isolation of fungi from Heterodera glycines and in vitro bioassays for their antagonism to eggs. J. Nematol. 1990, 22, 532–537. [Google Scholar] [PubMed]
- Langen, G.; Beißmann, B.; Reisener, H.J.; Kogel, K. A β-1,3-d-endo-mannanase from culture filtrates of the hyperparasites Verticillium lecanii and Aphanocladium album that specifically lyses the germ pore plug from uredospores of Puccinia graminis f.sp. tritici. Can. J. Bot. 1992, 70, 853–860. [Google Scholar] [CrossRef]
- Askary, H.; Benhamou, N.; Brodeur, J. Ultrastructural and cytochemical investigations of the antagonistic effect of Verticillium lecanii on cucumber powdery mildew. Phytopathology 1996, 87, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, M.A.; Hijwegen, T.; Zadoks, J.C. Glasshouse experiments on biocontrol of cucumber powdery mildew (Sphaerotheca fuliginea) by the mycoparasites Verticillium lecanii and Sporothrix rugulosa. BiolControl 1996, 6, 353–360. [Google Scholar]
- Benhamou, N.; Brodeur, I. Evidence for antibiosis and induced host defence reactions in the interaction between Verticillium lecanii and Penicillium digitatum the causal agent of green mould. Phytopathology 2000, 90, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Zare, R. A revision of Verticillium sect. Prostrata. III. Generic classification. Nova Hedwigia 2001, 72, 329–337. [Google Scholar]
- Fenice, M.; Selbmann, L.; Zucconi, L.; Onofri, S. Production of extracellular enzymes by Antarctic fungal strains. Polar Biol. 1997, 17, 275–280. [Google Scholar] [CrossRef]
- Fenice, M.; Selbmann, L.; di Giambattista, R.; Federici, F. Chitinolytic activity at low temperature of an Antarctic strain (A3) of Verticillium cfr. lecanii. Res. Microbiol. 1998, 149, 289–300. [Google Scholar] [CrossRef]
- Moscatelli, D. Attività Chitinolitiche del Ceppo Antartico Verticillium cfr. Lecanii A3: Studio a Livello Biochimico e Molecolare e Possibili Ricadute Applicative. Ph.D. Thesis, University of Tuscia, Viterbo, Italy, 11 July 2003. [Google Scholar]
- Chet, I. Trichoderma—Application, mode of action, and potential as biocontrol agent in soilborne plant pathogenic fungi. In Innovative Approaches to Plant Disease Control; Chet, I., Ed.; J. Wiley & Sons: New York, NY, USA, 1997; pp. 137–160. [Google Scholar]
- Fenice, M.; Barghini, P.; Selbmann, L.; Federici, F. Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microb. Cell Fac. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Marin-Cervantes, M.; Matsumoto, Y.; Ramírez-Coutiño, L.; Rocha-Pino, Z.; Viniegra, G.; Shirai, K. Effect of moisture content in polyurethane foams as support for solid-substrate fermentation of Lecanicillium lecanii on the production profiles of chitinases. Process. Biochem. 2008, 43, 24–32. [Google Scholar] [CrossRef]
- Bruce, A.; Scrinivasan, U.; Staines, H.J.; Highley, T.L. Chitinase and laminarinase production in liquid culture by Trichoderma spp. and their role in biocontrol of wood decay fungi. Int. Biodeterior. Biodegrad. 1995, 4, 337–353. [Google Scholar] [CrossRef]
- Benhamou, N.; Chet, I. Hyphal interaction between Trichoderma harzianum and Rhizoctonia solani: Ultrastructure and gold cytochemistry of the mycoparasitic process. Phytopathology 1993, 83, 1062–1071. [Google Scholar] [CrossRef]
- Inbar, J.; Menendez, A.; Chet, I. Hyphal interaction between Trichoderma harzianum and Sclerotinia sclerotiorum and its role in biological control. Soil Biol. Biochem. 1996, 28, 757–763. [Google Scholar] [CrossRef]
- Calistru, C.; McLean, M.; Berjak, P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. Mycopathologia 1997, 139, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; Isola, D.; Fenice, M.; Zucconi, L.; Sterflinger, K.; Onofri, S. Potential extinction of Antarctic endemic fungal species as consequence of Global Warming. Sci. Total Environ. 2012, 438, 127–134. [Google Scholar] [CrossRef] [PubMed]









| Description | Query Coverage | Identity | Accession N° |
|---|---|---|---|
| Chitinase (Metarhizium album) | 95% | 42% | KHN99830.1 |
| Chitinase (Hirsutella thompsonii) | 94% | 44% | AIT18903.1 |
| Chitinase (H. thompsonii) | 94% | 44% | AIT18889.1 |
| Endochitinase 33 (Tolypocladium ophioglossoide) | 94% | 43% | KND91481.1 |
| Chitinase 33 (Trichoderma virens) | 94% | 41% | ACJ04784.1 |
| Glycoside hydrolase family 18 (T. reesei) | 94% | 39% | XP_006961069.1 |
| Glycoside hydrolase family 18 (T. atroviride) | 94% | 42% | XP_013947241.1 |
| Endochitinase (T. atroviride) | 94% | 42% | ABO38127.1 |
| Glycoside hydrolase family 18 (T. virens) | 94% | 41% | XP_013958614.1 |
| Chitinase (T. virens) | 94% | 41% | ABP96986.1 |
| Chitinase 3 (Escovopsis weberi) | 94% | 36% | KOS22932.1 |
| Putative Chitinase (Torrubiella hemipterigena) | 93% | 50% | CEJ90418.1 |
| Related to endochitinase (Claviceps purpurea) | 93% | 49% | CCE32650.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenice, M. The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes. Molecules 2016, 21, 447. https://doi.org/10.3390/molecules21040447
Fenice M. The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes. Molecules. 2016; 21(4):447. https://doi.org/10.3390/molecules21040447
Chicago/Turabian StyleFenice, Massimiliano. 2016. "The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes" Molecules 21, no. 4: 447. https://doi.org/10.3390/molecules21040447
APA StyleFenice, M. (2016). The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes. Molecules, 21(4), 447. https://doi.org/10.3390/molecules21040447