Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of the Liquid and Solid SMEDDS
2.2. Dispersibility Test
2.3. Characterization of Solid SMEDDS
2.4. In Vitro Release Study
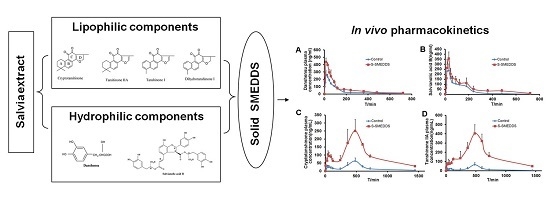
2.5. Bioavailability Study
3. Materials and Methods
3.1. Ethics Statement
3.2. Reagents and Chemicals
3.3. Preparation of Salvia Extract
3.4. Solubility Studies
3.5. Phase Diagram Construction
3.6. Screening for Solid Carriers
3.7. Preparation of a S-SMEDDS Containing a Multicomponent Mixture of Salvia Extract
3.8. Dispersibility Test
3.9. Determination of Droplet Size and Zeta Potential
3.10. Determination of Drug Content
3.11. Characterization of the Solid SMEDDS
3.12. In Vitro Release Assay
3.13. Bioavailability Study
3.14. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, L.; Ding, G.; Lin, H.; Cheng, H.; Kong, Y.; Wei, Y.; Fang, X.; Liu, R.; Wang, L.; Chen, X.; et al. Transcriptome analysis of medicinal plant Salvia miltiorrhiza and identification of genes related to tanshinone biosynthesis. PLoS ONE 2013, 8, e80464. [Google Scholar]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Jeon, B.H.; Kim, Y.C.; Lee, S.H.; Sohn, D.H.; Seo, G.S. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int. Immunopharmacol. 2013, 16, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, Y.; Li, W.; Cong, J.; Zhou, Y.; Ng, E.H.; Wu, X. Effects of tanshinone on hyperandrogenism and the quality of life in women with polycystic ovary syndrome: Protocol of a double-blind, placebo-controlled, randomised trial. BMJ Open 2013, 3, e003646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Woo, E.R.; Lee, H.W.; Park, H.R.; Kim, H.N.; Jung, Y.K.; Choi, J.Y.; Chae, S.W.; Kim, H.R.; Chae, H.J. The correlation of Salvia miltiorrhiza extract-induced regulation of osteoclastogenesis with the amount of components tanshinone I, tanshinone IIA, cryptotanshinone, and dihydrotanshinone. Immunopharmacol. Immunotoxicol. 2008, 30, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, X.; Liang, X.; Xiang, L.; Zhang, W. Metabolomic strategy to study therapeutic and synergistic effects of tanshinone IIA, salvianolic acid B and ginsenoside Rb1 in myocardial ischemia rats. J. Ethnopharmacol. 2011, 134, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Wang, T.; Wang, W.; Zhang, L.; Li, C.; Yu, P.; Wang, H.; Fu, F. Down-regulation of P-glycoprotein expression contributes to an increase in Danshensu accumulation in the cerebral ischemia/reperfusion brain. Mol. Med. Rep. 2012, 5, 812–816. [Google Scholar] [PubMed]
- Hao, H.; Wang, G.; Cui, N.; Li, J.; Xie, L.; Ding, Z. Pharmacokinetics, absorption and tissue distribution of tanshinone IIA solid dispersion. Planta Med. 2006, 72, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, M.; Guan, S.; Bi, H.C.; Pan, Y.; Duan, W.; Chan, S.Y.; Chen, X.; Hong, Y.H.; Bian, J.S.; et al. A mechanistic study of the intestinal absorption of cryptotanshinone, the major active constituent of Salvia miltiorrhiza. J. Pharmacol. Exp. Ther. 2006, 317, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chow, M.S.; Zuo, Z. Effect of sodium caprate on the oral absorptions of danshensu and salvianolic acid B. Int. J. Pharm. 2009, 379, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Bi, H.C.; Zhong, G.P.; Chen, X.; Zuo, Z.; Zhao, L.Z.; Gu, L.Q.; Liu, P.Q.; Huang, Z.Y.; Zhou, S.F.; et al. Pharmacokinetic characterization of hydroxylpropyl-β-cyclodextrin-included complex of cryptotanshinone, an investigational cardiovascular drug purified from Danshen (Salvia miltiorrhiza). Xenobiotica 2008, 38, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, P.; Liu, J.P.; Zhang, W.L.; Yang, J.K.; Fan, Y.Q. Novel Tanshinone II A ternary solid dispersion pellets prepared by a single-step technique: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, P.; Liu, J.P.; Yang, J.K.; Zhang, W.L.; Fan, Y.Q.; Kan, S.L.; Cui, Y.; Zhang, W.J. Bioavailability and foam cells permeability enhancement of Salvianolic acid B pellets based on drug-phospholipids complex technique. Eur. J. Pharm. Biopharm. 2013, 83, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Gong, T.; Zuo, J.; Liu, J.; Zhao, D.; Zhang, Z. Enhanced oral bioavailability of salvianolic acid B by phospholipid complex loaded nanoparticles. Die Pharm. 2008, 63, 661–666. [Google Scholar]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, C.M. Lipid-based formulations for intestinal lymphatic delivery. Eur. J. Pharm. Sci. 2002, 15, 405–415. [Google Scholar] [CrossRef]
- Hauss, D.J.; Mehta, S.C.; Radebaugh, G.W. Targeted lymphatic transport and modified systemic distribution of CI-976, a lipophilic lipid-regulator drug, via a formulation approach. Int. J. Pharm. 1994, 108, 85–93. [Google Scholar] [CrossRef]
- Patel, A.R.; Vavia, P.R. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007, 9, E344–E352. [Google Scholar] [CrossRef] [PubMed]
- Tuleu, C.; Newton, M.; Rose, J.; Euler, D.; Saklatvala, R.; Clarke, A.; Booth, S. Comparative bioavailability study in dogs of a self-emulsifying formulation of progesterone presented in a pellet and liquid form compared with an aqueous suspension of progesterone. J. Pharm. Sci. 2004, 93, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, S.; Khan, M. Controlled release of a self-emulsifying formulation from a tablet dosage form: Stability assessment and optimization of some processing parameters. Int. J. Pharm. 2006, 315, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid Interface Sci. 2009, 330, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, G.; Gu, J.C.; Xu, C.H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.R.; Shao, J. Self-nanoemulsifying drug delivery systems (SMEDDS) for oral delivery of protein drugs: I. Formulation development. Int. J. Pharm. 2008, 362, 2–9. [Google Scholar] [PubMed]
- Li, P.; Ghosh, A.; Wagner, R.F.; Krill, S.; Joshi, Y.M.; Serajuddin, A.T. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int. J. Pharm. 2005, 288, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.J.; Lebinson, R.S. Lyophilized Emulsion Composition and Method. EP0211257 (A2), 4 September 1990. [Google Scholar]
- Dixit, A.R.; Rajput, S.J.; Patel, S.G. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS PharmSciTech 2010, 11, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Zeng, J.Q.; Liu, L.M.; Jin, X.S. Extraction of three tanshinones from the root of Salvia miltiorrhiza Bunge by supercritical carbon dioxide fluid and their analysis with high performance liquid chromatography. Chin. J. Chromatogr. 2002, 20, 40–42. (In Chinese) [Google Scholar]
- Chen, Z.Q.; Liu, Y.; Zhao, J.H.; Wang, L.; Feng, N.P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int. J. Nanomed. 2012, 7, 1115–1125. [Google Scholar]
- Nekkanti, V.; Karatgi, P.; Prabhu, R.; Pillai, R. Solid self-microemulsifying formulation for candesartan cilexetil. AAPS PharmSciTech 2010, 11, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bi, G.W.; Long, X.Y.; Yu, Z.M.; Yang, W.S.; Feng, L.P. The preparation and the in vitro release of OANO-1 microspheres. Chin. J. Chin. Mater. Med 2005, 30, 992–994. (In Chinese) [Google Scholar]
- Sample Availability: Samples of the solid SMEDDS containing a multicomponent mixture extracted from Salvia miltiorrhiza are available from the authors.








| Vehicle | Danshensu (mg·g−1) | Salvianolic Acid B (mg·g−1) | Dihydrotanshinone I (mg·g−1) | Cryptotanshinone (mg·g−1) | Tanshinone I (mg·g−1) | Tanshinone IIA (mg·g−1) | |
|---|---|---|---|---|---|---|---|
| Oils | IPM | - | - | 0.388 ± 0.035 | 0.226 ± 0.013 | 1.757 ± 0.062 | 4.525 ± 0.026 |
| Maisine 35-1 | 3.493 ± 0.015 | 2.350 ± 0.136 | 0.513 ± 0.014 | 0.101 ± 0.021 | 1.365 ± 0.042 | 4.123 ± 0.018 | |
| Labrafac | - | - | - | 0.301 ± 0.038 | 1.191 ± 0.017 | 3.783 ± 0.121 | |
| Peceol | - | - | 0.377 ± 0.012 | - | 1.179 ± 0.023 | 2.742 ± 0.029 | |
| Surfactants | Cremophor RH40 | 1.188 ± 0.023 | 3.439 ± 0.103 | - | 0.399 ± 0.047 | 1.497 ± 0.037 | 4.094 ± 0.079 |
| Labrafil M 1944 | - | - | 0.442 ± 0.110 | - | 1.283 ± 0.017 | 3.805 ± 0.169 | |
| Solutol HS15 | 0.677 ± 0.126 | - | 0.136 ± 0.009 | 0.197 ± 0.027 | 1.527 ± 0.028 | 3.760 ± 0.321 | |
| Labrasol | 1.629 ± 0.220 | 1.783 ± 0.209 | 0.307 ± 0.052 | 0.127 ± 0.017 | 1.405 ± 0.175 | 3.323 ± 0.261 | |
| Tween-80 | 1.624 ± 0.158 | 1.155 ± 0.067 | - | 0.204 ± 0.046 | - | 3.241 ± 0.333 | |
| Gelucire 44/14 | 0.813 ± 0.216 | 0.733 ± 0.08 | 0.428 ± 0.099 | - | 1.139 ± 0.114 | 2.035 ± 0.07 | |
| Cosurfactants | Transcutol P | 2.410 ± 0.202 | 6.480 ± 0.258 | 0.888 ± 0.036 | 0.663 ± 0.114 | 2.367 ± 0.136 | 3.579 ± 0.133 |
| Ethanol | 0.961 ± 0.151 | 3.325 ± 0.143 | 0.499 ± 0.02 | - | 1.229 ± 0.108 | 2.022 ± 0.087 | |
| PEG400 | 0.309 ± 0.037 | 4.004 ± 0.027 | - | 0.260 ± 0.041 | 1.277 ± 0.117 | 2.429 ± 0.125 | |
| Formulation Composition (35:45:20 w/w/w) | Droplet Size (nm) | Polydispersibility | Solubility | |||||
|---|---|---|---|---|---|---|---|---|
| Danshensu (mg·g−1) | Salvianolic Acid B (mg·g−1) | Dihydrotanshinone I (mg·g−1) | Tanshinone I (mg·g−1) | Cryptotanshinone (mg·g−1) | Tanshinone IIA (mg·g−1) | |||
| A | 64.60 | 0.136 | 0.34 ± 0.07 | 0.49 ± 0.07 | 1.50 ± 0.29 | 1.46 ± 0.12 | 4.64 ± 0.29 | 5.14 ± 0.12 |
| B | 67.20 | 0.176 | 0.57 ± 0.12 | 0.62 ± 0.10 | 1.43 ± 0.13 | 1.39 ± 0.15 | 4.28 ± 0.28 | 5.30 ± 0.18 |
| C | 102.30 | 0.264 | 1.75 ± 0.32 | 2.03 ± 0.05 | 1.25 ± 0.09 | 1.45 ± 0.30 | 4.05 ± 0.39 | 4.59 ± 0.29 |
| D | 91.56 | 0.390 | 1.51 ± 0.09 | 2.31 ± 0.17 | 1.33 ± 0.20 | 1.37 ± 0.16 | 4.60 ± 0.40 | 4.67 ± 0.23 |
| E | 49.20 | 0.073 | 1.37 ± 0.11 | 1.11 ± 0.20 | 1.42 ± 0.36 | 1.40 ± 0.24 | 4.65 ± 0.32 | 5.16 ± 0.17 |
| Carrier | Danshensu (mg·g−1) | Salvianolic Acid B (mg·g−1) | Dihydrotanshinone I (mg·g−1) | Tanshinone I (mg·g−1) | Cryptotanshinone (mg·g−1) | Tanshinone IIA (mg·g−1) |
|---|---|---|---|---|---|---|
| Mannitol | 1.61 ± 0.06 | 13.88 ± 0.30 | 0.20 ± 0.09 | 0.21 ± 0.06 | 0.64 ± 0.14 | 1.03 ± 0.14 |
| Lactose | 1.47 ± 0.12 | 13.53 ± 0.19 | 0.20 ± 0.04 | 0.20 ± 0.01 | 0.60 ± 0.10 | 0.94 ± 0.05 |
| Dextran-40 | 1.63 ± 0.07 | 14.30 ± 0.19 | 0.21 ± 0.01 | 0.23 ± 0.03 | 0.71 ± 0.12 | 1.14 ± 0.12 |
| Glucose | 1.49 ± 0.11 | 13.45 ± 0.29 | 0.18 ± 0.02 | 0.20 ± 0.02 | 0.59 ± 0.04 | 0.89 ± 0.01 |
| Formulation | Cmax (ng·mL−1) | Tmax (h) | T1/2 (h) | AUC0–t (μg·h·mL−1) | Relative Bioavailability (%) |
|---|---|---|---|---|---|
| Danshensu, suspension | 278.84 ± 32.52 | 0.29 ± 0.10 | 3.39 ± 0.95 | 471.51 ± 52.11 | |
| Danshensu, S-SMEDDS | 451.82 ± 30.22 *** | 0.33 ± 0.13 | 3.56 ± 1.24 | 822.43 ± 91.70 *** | 174.4 |
| Salvianolic acid B, suspension | 285.09 ± 59.04 | 0.38 ± 0.14 | 1.95 ± 0.87 | 484.02 ± 65.14 | |
| Salvianolic acid B, S-SMEDDS | 369.41 ± 65.85 * | 0.46 ± 0.10 | 3.63 ± 1.001 * | 801.23 ± 148.55 ** | 165.5 |
| Cryptotanshinone, suspension | 63.38 ± 12.05 | 8.00 ± 0.00 | 3.33 ± 2.29 | 7.92 ± 2.31 | |
| Cryptotanshinone, S-SMEDDS | 270.42 ± 63.47 *** | 8.40 ± 0.89 | 5.02 ± 1.78 | 39.52 ± 9.91 *** | 499.3 |
| Tanshinone IIA, suspension | 65.83 ± 20.24 | 8.00 ± 0.00 | 4.11 ± 2.06 | 6.93 ± 0.92 | |
| Tanshinone IIA, S-SMEDDS | 403.34 ± 90.71 *** | 8.00 ± 0.00 | 4.71 ± 0.87 | 65.04 ± 14.07 *** | 938.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, X.; Liu, X.; Di, L.; Zu, Q. Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza. Molecules 2016, 21, 456. https://doi.org/10.3390/molecules21040456
Bi X, Liu X, Di L, Zu Q. Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza. Molecules. 2016; 21(4):456. https://doi.org/10.3390/molecules21040456
Chicago/Turabian StyleBi, Xiaolin, Xuan Liu, Liuqing Di, and Qiang Zu. 2016. "Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza" Molecules 21, no. 4: 456. https://doi.org/10.3390/molecules21040456
APA StyleBi, X., Liu, X., Di, L., & Zu, Q. (2016). Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza. Molecules, 21(4), 456. https://doi.org/10.3390/molecules21040456