Photoactivatable Caged Prodrugs of VEGFR-2 Kinase Inhibitors
Abstract
:1. Introduction
2. Results
2.1. Molecular Modeling
2.2. Synthesis
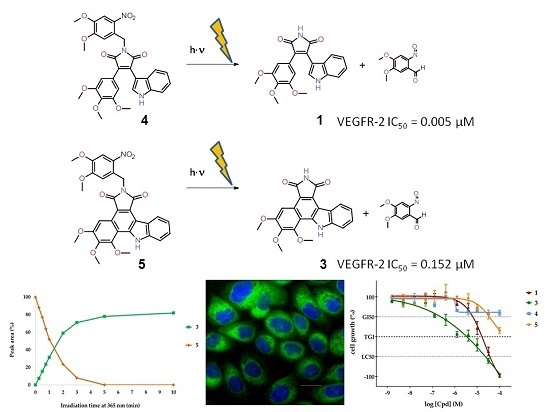
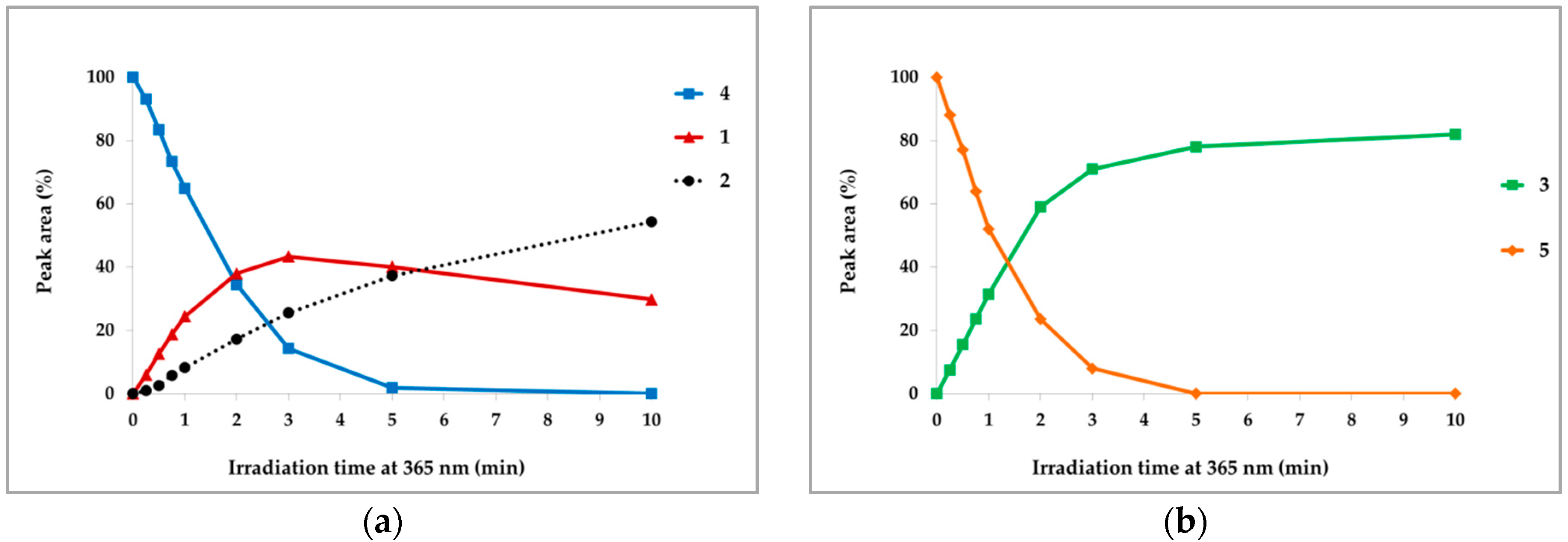
2.3. Photochemical Characterization
2.4. Enzyme Assays
2.5. Cellular Assays
3. Discussion
4. Materials and Methods
4.1. Molecular Modeling
4.2. Chemistry
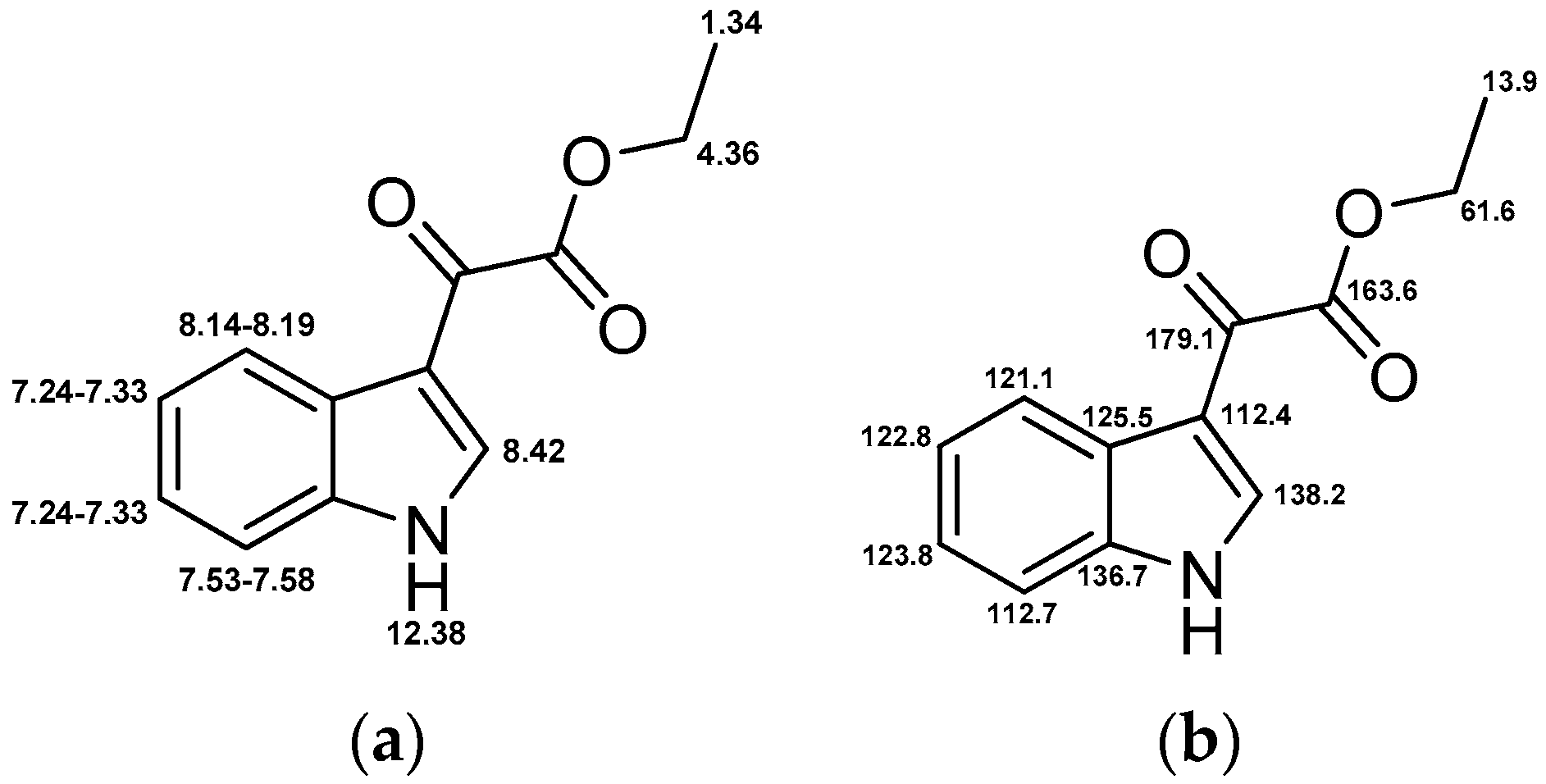
4.3. Chemical Synthesis and Characterization
4.3.1. 3-(1H-Indol-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-pyrrole-2,5-dione (1)
4.3.2. 5,6,7-Trimethoxybenzo[a]pyrrolo[3,4-c]carbazole-1,3(2H,8H)-dione (3)
4.3.3. 1-(4,5-Dimethoxy-2-nitrobenzyl)-3-(1H-indol-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-pyrrole-2,5-dione (4)
4.3.4. 2-(4,5-Dimethoxy-2-nitrobenzyl)-5,6,7-trimethoxybenzo[a]pyrrolo[3,4-c]carbazole-1,3(2H,8H)- dione (5)
4.3.5. 2-(3,4,5-Trimethoxyphenyl)acetamide (7)
4.3.6. Ethyl 2-(1H-indol-3-yl)-2-oxoacetate (10)
4.4. Photochemical Characterization
4.4.1. UV/Vis Absorption Spectra
4.4.2. Fluorescence Emission Spectra
4.4.3. UV Stability
4.4.4. Photoactivation
4.5. Kinase Assays
4.5.1. Determination of IC50 Values
4.5.2. Kinase Profiling
4.6. CellularAssays
4.6.1. Cell Culture
4.6.2. Proliferation Assays
4.6.3. Cell Staining
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMNB | 4,5-dimethoxy-2-nitrobenzyl |
| e.g. | for example |
| HPLC | high performance liquid chromatography |
| LC | liquid chromatography |
| MS | mass spectrometry |
| PBS | phosphate buffered saline |
| PPG | photoremovable protecting group |
| resp. | respectively |
| UV | ultraviolet |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Hall, R.D.; Le, T.M.; Haggstrom, D.E.; Gentzler, R.D. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl. Lung Cancer Res. 2015, 4, 515–523. [Google Scholar] [PubMed]
- Cella, D.; Beaumont, J.L. Pazopanib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Schwarz, R.E. Profile of nintedanib in the treatment of solid tumors: The evidence to date. Onco. Targets Ther. 2015, 8, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shen, J.; Vinores, S.A. Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS ONE 2011, 6, e21411. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, D.; Han, Y.-S.; Baek, M.J. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann. Surg. Treat. Res. 2015, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hartsough, E.J.; Meyer, R.D.; Chitalia, V.; Jiang, Y.; Marquez, V.E.; Zhdanova, I.V.; Weinberg, J.; Costello, C.E.; Rahimi, N. Lysine methylation promotes VEGFR-2 activation and angiogenesis. Sci. Signal. 2013, 6, ra104. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Bronte, G.; Sortino, G.; Papadimitriou, K.; Passiglia, F.; Fiorentino, E.; Marogy, G.; Russo, A.; Peeters, M. The role of targeted therapy for gastrointestinal tumors. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 875–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, S.; Man Wong, K.; Jimeno, A. Nintedanib, a novel triple angiokinase inhibitor for the treatment of non-small cell lung cancer. Drugs Today 2015, 51, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.; Ignatov, A. The Role of Targeted Agents in the Treatment of Metastatic Breast Cancer. Breast Care 2010, 5, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.W. Dosing strategies and optimization of targeted therapy in advanced renal cell carcinoma. J. Oncol. Pharm. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Stępniak, J. The safety of regorafenib for the treatment of gastrointestinal stromal tumors. Expert Opin. Drug Saf. 2016, 15, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Ferrari, S.M.; Politti, U.; Mazzi, V.; Miccoli, M.; Materazzi, G.; Antonelli, A.; Ulisse, S.; Fallahi, P.; Miccoli, P. Aggressive thyroid cancer: targeted therapy with sorafenib. Min. Endocrinol. 2015, in press. [Google Scholar]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rini, B.I.; Small, E.J. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol. 2005, 23, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov. Today 2016, 21, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.E.; Roman, I.I.; Constantin, A.-M.; Mihu, C.M.; Tătaru, A.D. VEGF involvement in psoriasis. Clujul Med. 2015, 88, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.W.; Bressler, S.B. Long-term follow-up of vascular endothelial growth factor inhibitor therapy for neovascular age-related macular degeneration. Curr. Opin. Ophthalmol. 2013, 24, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sweeney, C.; Walsh, C.; Rooney, P.; McCormick, J.; Veale, D.J.; Fearon, U. Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann. Rheum. Dis. 2013, 72, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Gomez-Arroyo, J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am. J. Respir. Cell Mol. Biol. 2014, 51, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Man, X.-Y.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Zheng, M. Targeting VEGF/VEGFR in the treatment of psoriasis. Discov. Med. 2014, 18, 97–104. [Google Scholar] [PubMed]
- Peifer, C.; Krasowski, A.; Hämmerle, N.; Kohlbacher, O.; Dannhardt, G.; Totzke, F.; Schächtele, C.; Laufer, S. Profile and molecular modeling of 3-(indole-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-pyrrole-2,5-dione (1) as a highly selective VEGF-R2/3 inhibitor. J. Med. Chem. 2006, 49, 7549–7553. [Google Scholar] [CrossRef] [PubMed]
- Peifer, C.; Stoiber, T.; Unger, E.; Totzke, F.; Schächtele, C.; Marmé, D.; Brenk, R.; Klebe, G.; Schollmeyer, D.; Dannhardt, G. Design, synthesis, and biological evaluation of 3,4-diarylmaleimides as angiogenesis inhibitors. J. Med. Chem. 2006, 49, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Heckel, A. Biologically active molecules with a “light switch”. Angew. Chem. Int. Ed. 2006, 45, 4900–4921. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Meth. 2007, 4, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, H.; Wang, L.; Liu, Y.; Knapp, S.; Liu, Q.; Gray, N.S. Exploration of type II binding mode: A privileged approach for kinase inhibitor focused drug discovery? ACS Chem. Biol. 2014, 9, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Horbert, R.; Pinchuk, B.; Davies, P.; Alessi, D.; Peifer, C. Photoactivatable Prodrugs of Antimelanoma Agent Vemurafenib. ACS Chem. Biol. 2015, 10, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morckel, A.R.; Lusic, H.; Farzana, L.; Yoder, J.A.; Deiters, A.; Nascone-Yoder, N.M. A photoactivatable small-molecule inhibitor for light-controlled spatiotemporal regulation of Rho kinase in live embryos. Development 2011, 139, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H.; Forbush, B.; Hoffman, J.F. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: Utilization by the Na:K pump of human red blood cell ghosts. Biochemistry 1978, 17, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Corrie, J.E.; Furuta, T.; Givens, R.; Yousef, A.L.; Goeldner, M. Photoremovable Protecting Groups Used for the Caging of Biomolecules. In Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged Biomolecules; Goeldner, M., Givens, R., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–94. [Google Scholar]
- Sadovski, O.; Jaikaran, A.S.I.; Samanta, S.; Fabian, M.R.; Dowling, R.J.O.; Sonenberg, N.; Woolley, G.A. A collection of caged compounds for probing roles of local translation in neurobiology. Bioorg. Med. Chem. 2010, 18, 7746–7752. [Google Scholar] [CrossRef] [PubMed]
- Bliman, D.; Nilsson, J.R.; Kettunen, P.; Andréasson, J.; Grøtli, M. A Caged Ret Kinase Inhibitor and its Effect on Motoneuron Development in Zebrafish Embryos. Sci. Rep. 2015, 5, 13109. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wan, Z.; Gao, Y.; Zheng, J.-S.; Wang, J.; Si, Y.-Y.; Chen, X.; Qi, H.; Liu, L.; Liu, W. Total chemical synthesis of photoactivatable proteins for light-controlled manipulation of antigen–antibody interactions. Chem. Sci. 2016, 7, 1891–1895. [Google Scholar] [CrossRef]
- Janett, E.; Bernardinelli, Y.; Müller, D.; Bochet, C.G. Synthesis of FMRFaNV, a Photoreleasable Caged Transmitter Designed to Study Neuron–Glia Interactions in the Central Nervous System. Bioconjugate Chem. 2015, 26, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.W.; Guo, Z.-F.; Liang, F.-S. Light Control of Cellular Processes by Using Photocaged Abscisic Acid. ChemBioChem 2015, 16, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Horbert, R. Photoactivatable Kinase Inhibitors. Ph.D. Thesis, Christian-Albrechts-University of Kiel, Kiel, Germany, 2015. [Google Scholar]
- Flash Plate-Based Protein Kinase Assay Protocol (33 PanQinase® Assay); ProQinase GmbH: Freiburg im Breisgau, Germany, 2012.
- Hastie, C.J.; McLauchlan, H.J.; Cohen, P. Assay of protein kinases using radiolabeled ATP: A protocol. Nat. Protoc. 2006, 1, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Yi, Z.; Cho, S.-G.; Luo, J.; Pandey, M.K.; Aggarwal, B.B.; Liu, M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008, 68, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Costa, R.; Froufe, H.J.C.; Calhelha, R.C.; Peixoto, D.; Ferreira, I.C.F.R.; Abreu, R.M.V.; Soares, R.; Queiroz, M.-J.R.P. 1-Aryl-3-[4-(thieno[3,2-d]pyrimidin-4-yloxy)phenyl]ureas as VEGFR-2 tyrosine kinase inhibitors: Synthesis, biological evaluation, and molecular modelling studies. Biomed. Res. Int. 2013, 2013, 154856. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Kumar, S.; Alhaider, A.A. α-Santalol inhibits the angiogenesis and growth of human prostate tumor growth by targeting vascular endothelial growth factor receptor 2-mediated AKT/mTOR/P70S6K signaling pathway. Mol. Cancer 2013, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.S.; Koszelak, M.; Liu, J.; Lawrence, D.S. A Caged Protein Kinase Inhibitor. J. Am. Chem. Soc. 1998, 1998, 7145–7146. [Google Scholar] [CrossRef]
- Zindler, M.; Pinchuk, B.; Renn, C.; Horbert, R.; Döbber, A.; Peifer, C. Design, Synthesis, and Characterization of a Photoactivatable Caged Prodrug of Imatinib. ChemMedChem 2015, 10, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L. Encyclopedia of Entomology, 2nd ed.; Springer: Dordrecht, The Netherlands; London, UK, 2008. [Google Scholar]
- Goswami, P.P.; Syed, A.; Beck, C.L.; Albright, T.R.; Mahoney, K.M.; Unash, R.; Smith, E.A.; Winter, A.H. BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. J. Am. Chem. Soc. 2015, 137, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; Aujard, I.; le Saux, T.; Maurin, S.; Beaupierre, S.; Baudin, J.-B.; Jullien, L. Coumarinylmethyl Caging Groups with Redshifted Absorption. Chem. Eur. J. 2013, 19, 17494–17507. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Waynant, R.W.; Ilev, I.K.; Wu, X.; Barna, L.; Smith, K.; Heckert, R.; Gerst, H.; Anders, J.J. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005, 36, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Bort, G.; Gallavardin, T.; Ogden, D.; Dalko, P.I. From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches. Angew. Chem. Int. Ed. 2013, 52, 4526–4537. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2014-2: Schrödinger Suite 2014-2 Protein Preparation Wizard; Epik, version 2.8; Impact, version 6.3; Prime, version 3.6; Schrödinger, LLC: New York, NY, USA, 2014.
- Schrödinger Release 2014-1: Epik, version 2.7; Schrödinger, LLC: New York, NY, USA, 2014.
- Small-Molecule Drug Discovery Suite 2014-2: PrimeX, version 2.4; Schrödinger, LLC: New York, NY, USA, 2014.
- Schrödinger Release 2014-1: MacroModel, version 10.3; Schrödinger, LLC: New York, NY, USA, 2014.
- Schrödinger Release 2014-1: LigPrep, version 2.9; Schrödinger, LLC: New York, NY, USA, 2014.
- Schrödinger Release 2014-1: ConfGen, version 2.7; Schrödinger, LLC: New York, NY, USA, 2014.
- Small-Molecule Drug Discovery Suite 2014-1: Glide, version 6.2; Schrödinger, LLC: New York, NY, USA, 2014.
- Sample Availability: Samples of the compounds 1, 3, 4 and 5 are available from the authors.













| Compound | VEGFR-2 IC50 (µM) |
|---|---|
| 1 | 0.005 |
| 3 | 0.152 |
| 4 | 4.590 |
| 5 | 44.80 |
| Compound | GI50 without Irradiation (µM) | GI50 after Irradiation (µM) |
|---|---|---|
| 1 | 6.4 | 2.9 |
| 3 | 0.2 | 0.4 |
| 4 | not reached | 0.1 |
| 5 | 34.6 | 0.2 |
| Compound | Filter Set | Exciter (nm) (Center/Bandwidth) | Emitter (nm) (Center/Bandwidth) |
|---|---|---|---|
| DAPI | DAPI | 377/50 | 447/60 |
| 3 | GFP | 472/30 | 520/35 |
| 5 | YFP | 500/24 | 542/27 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinchuk, B.; Horbert, R.; Döbber, A.; Kuhl, L.; Peifer, C. Photoactivatable Caged Prodrugs of VEGFR-2 Kinase Inhibitors. Molecules 2016, 21, 570. https://doi.org/10.3390/molecules21050570
Pinchuk B, Horbert R, Döbber A, Kuhl L, Peifer C. Photoactivatable Caged Prodrugs of VEGFR-2 Kinase Inhibitors. Molecules. 2016; 21(5):570. https://doi.org/10.3390/molecules21050570
Chicago/Turabian StylePinchuk, Boris, Rebecca Horbert, Alexander Döbber, Lydia Kuhl, and Christian Peifer. 2016. "Photoactivatable Caged Prodrugs of VEGFR-2 Kinase Inhibitors" Molecules 21, no. 5: 570. https://doi.org/10.3390/molecules21050570