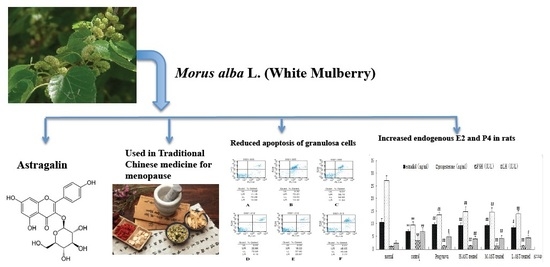
Astragalin, a Flavonoid from Morus alba (Mulberry) Increases Endogenous Estrogen and Progesterone by Inhibiting Ovarian Granulosa Cell Apoptosis in an Aged Rat Model of Menopause
Abstract
:1. Introduction
2. Results
2.1. AST Does Not Alter Uterine, Ovarian and Pituitary Indices in Aged Female Rats
2.2. AST Reduces Apoptosis of Ovarian GCs in Aged Female Rats
2.3. AST Increases Hormone Production in Aged Female Rats
2.4. AST Increases GC Proliferation in Cultured GCs
2.5. AST Increases GC Hormone Production and Secretion in Cultured GCs
2.6. AST Reduced Apoptosis in Cultured GCs
2.7. AST Alters the Ratio of Bcl-2 and Bax Proteins in Cultured GCs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Effects of AST on GC Apoptosis In Vivo
4.2.1. Naturally Aged Rat Model of Menopause
4.2.2. Experimental Design
4.2.3. Histological Analysis
4.2.4. Apoptosis Assay
+ the number of normal cells) × 100% as described by the manufacturer
4.2.5. Radioimmunoassay of Hormone Levels
4.3. Effects of AST on GC Apoptosis In Vitro
4.3.1. Ovarian Granulosa Cell Culture
4.3.3. Radioimmunoassay Measurements of Estradiol and Progesterone in Cultured GCs
4.3.4. Annexin V Apoptosis Assay
4.3.5. Western Blot Assays
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.; Santoro, N. The reproductive endocrinology of the menopausal transition. Steroids 2011, 76, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Randolph, J.F., Jr. Reproductive hormones and the menopause transition. Obstet. Gynecol. Clin. N. Am. 2011, 38, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Sui, H.S.; Han, Z.B.; Li, W.; Luo, M.J.; Tan, J.H. Apoptosis in granulosa cells during follicle atresia: Relationship with steroids and insulin-like growth factors. Cell Res. 2004, 14, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F.; Carbone, M.C. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F. The aging ovary—The poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Heckbert, S.R.; Kaplan, R.C.; Weiss, N.S. Risk of recurrent coronary events in relation to use and recent initiation of postmenopausal hormone therapy. Arch. Intern. Med. 2001, 161, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Grady, D.; Bush, T. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Patel, A.V.; Calle, E.E.; Jacob, E.J.; Thun, M.J. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA 2001, 285, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2003, 288, 321–333. [Google Scholar]
- United States Preventive Services Task Force. Postmenopausal hormone replacement therapy for primary prevention of chronic conditions: Recommendations and rationale. Ann. Intern. Med. 2002, 137, 834–839. [Google Scholar]
- Peng, W.; Sibbritt, D.W.; Hickman, L.; Kong, X.; Yang, L.; Adams, J. A critical review of traditional Chinese medicine use amongst women with menopausal symptoms. Climacteric 2014, 17, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zheng, S.Z.; Lu, Y.; Liu, D.; Ma, H.; Mahady, G.B. Herbal formula Menoprogen alters insulin-like growth factor-1 and insulin-like growth factor binding protein-1 levels in the serum and ovaries of an aged female rat model of menopause. Menopause 2015, 22, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, Y.; Ma, H.; Mahady, G.B. A pilot observational study to assess the safety and efficacy of Menoprogen for the management of menopausal symptoms in Chinese women. J. Altern. Complement. Med. 2009, 15, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, J.; Wen, Z.; Zha, Q.; Nie, G.; Huang, X.; Zhang, C.; Lu, A.; Jiang, M.; Wang, X. Effect of combining therapy with traditional Chinese medicine-based psychotherapy and herbal medicines in women with menopausal syndrome: A randomized controlled clinical trial. Evid. Based Complement. Altern. Med. 2012, 35, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chung, M.H.; Lu, Y.; Hattori, M. Estrogenic effects of the herbal formula, Menoprogen, in ovariectomized rats. Biol. Pharm. Bull. 2010, 33, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Ma, H.; Yu, L.; Min, W.; Liu, D. Novel mechanism of action for Menoprogen, a traditional Chinese formula for the treatment of menopause. J. Women’s Health 2013, 22. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Lu, Y.; Tan, B.J.; Xu, L.; Lawal, T.O.; Mahady, G.B.; Liu, D. Menoprogen, a TCM herbal formula for menopause, increases endogenous E2 in an aged rat model of menopause by reducing ovarian granulosa cell apoptosis. BioMed Res. Int. 2016; in press. [Google Scholar]
- Wang, G.L.; Wei, M.; Wang, J.; Lu, Y.; Mahady, G.B.; Liu, D. High-performance liquid chromatography with photodiode array detector (HPLC-PAD) quality control of Menoprogen, a traditional Chinese Medicine (TCM) formula used for the management of menopause. Int. J. Med. Plant Res. 2013, 2, 146–151. [Google Scholar]
- Channing, C.P.; Ledwitz-Rigby, F. Methods in Enzymology; Hardman, J.G., O’Malley, B.W., Eds.; Academic Press: New York, NY, USA, 1975; Volume 39, Part D; pp. 183–185. [Google Scholar]
- Roberts, H.; Lethaby, A. Phytoestrogens for menopausal vasomotor symptoms: A Cochrane review summary. Maturitas 2014, 78, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Hajirahimkhan, A.; Dietz, B.M.; Bolton, J.L. Botanical modulation of menopausal symptoms: Mechanisms of action? Planta Med. 2013, 79, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Deepa, M.; Sureshkumar, T.; Satheeshkumar, P.K. Antioxidant rich Morus alba leaf extract induces apoptosis in human colon and breast cancer cells by the down-regulation of nitric oxide produced by inducible nitric oxide synthase. Nutr. Cancer 2013, 65, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kang, H.J.; Kim, S.Z. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch. Pharm Res. 2013, 36, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Han, J.; Ren, H.; Yang, W.; Zhang, X.; Zheng, Q.; Wang, D. Cardioprotective effects of astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid. Med. Cell. Longev 201 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Choi, Y.J.; Gong, J.H.; Shin, D.; Kang, M.K.; Kang, Y.H. Astragalin inhibits autophagy-associated airway epithelial fibrosis. Respir. Res. 2015, 16, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Gong, J.H.; Kang, M.K.; Lee, E.J.; Park, J.H.; Park, S.J.; Kang, Y.H. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm. Med. 2014, 14, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Liang, D.J.; Yang, Z.T. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. Int. Immunopharmacol. 2013, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Zhu, P.P.; Zhang, C.F. Influence of astragalin on proliferation and differentiation of mice’s osteoblast MC3T3-E1. Acta Chin. Med. Pharmacol. 2013, 41, 17–19. [Google Scholar]
- Perez, G.; Robles, R.; Knudson, C.M. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 1999, 21, 200–203. [Google Scholar] [PubMed]
- Matsuda-Minehata, F.; Inoue, N.; Goto, Y. The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. J. Reprod. Dev. 2006, 52, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringaoleifera Lam. J. Chromatogr. Sci. 2014, 52, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, S.W. Quantitative changes of polyphenolic compounds in mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev. Nutr. Food Sci. 2012, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liao, S.; Shen, W.; Liu, F.; Tang, C.; Chen, C.Y.; Sun, Y. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int. J. Mol. Sci. 2012, 13, 16544–16553. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.; Quintana, J.; Díaz, J.G.; Estévez, F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. Cancer Lett. 2011, 309, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Tagahara, K.; Suzuki, H.; Wu, R.Y.; Haruna, M.; Hall, I.H.; Huang, H.C.; Ito, K.; Iida, T.; Lai, J.S. Antitumor agents. 49 tricin, kaempferol-3-O-β-d-glucopyranoside and (+)-nortrachelogenin, antileukemic principles from Wikstroemiaindica. J. Nat. Prod. 1981, 44, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Moawad, A.; Owis, A.; AbouZid, S.; Ahmed, O. Flavonoids of Calligonumpolygonoides and their cytotoxicity. Pharm. Biol. 2016, 28. [Google Scholar] [CrossRef]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, M.B.; Hossain, M.; Rashid, M.; Sadik, G.; Nasrin, S.; et al. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morusalba L. (Moraceae). BMC Res. Notes 2013, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds astragalin are available from the authors.










© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Mahady, G.B.; Liu, D.; Zheng, Z.S.; Lu, Y. Astragalin, a Flavonoid from Morus alba (Mulberry) Increases Endogenous Estrogen and Progesterone by Inhibiting Ovarian Granulosa Cell Apoptosis in an Aged Rat Model of Menopause. Molecules 2016, 21, 675. https://doi.org/10.3390/molecules21050675
Wei M, Mahady GB, Liu D, Zheng ZS, Lu Y. Astragalin, a Flavonoid from Morus alba (Mulberry) Increases Endogenous Estrogen and Progesterone by Inhibiting Ovarian Granulosa Cell Apoptosis in an Aged Rat Model of Menopause. Molecules. 2016; 21(5):675. https://doi.org/10.3390/molecules21050675
Chicago/Turabian StyleWei, Min, Gail B. Mahady, Daniel Liu, Zhi S. Zheng, and Ye Lu. 2016. "Astragalin, a Flavonoid from Morus alba (Mulberry) Increases Endogenous Estrogen and Progesterone by Inhibiting Ovarian Granulosa Cell Apoptosis in an Aged Rat Model of Menopause" Molecules 21, no. 5: 675. https://doi.org/10.3390/molecules21050675
APA StyleWei, M., Mahady, G. B., Liu, D., Zheng, Z. S., & Lu, Y. (2016). Astragalin, a Flavonoid from Morus alba (Mulberry) Increases Endogenous Estrogen and Progesterone by Inhibiting Ovarian Granulosa Cell Apoptosis in an Aged Rat Model of Menopause. Molecules, 21(5), 675. https://doi.org/10.3390/molecules21050675