Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings
Abstract
:1. Introduction
2. Results
2.1. SEM, Profilometry and Topography Investigations
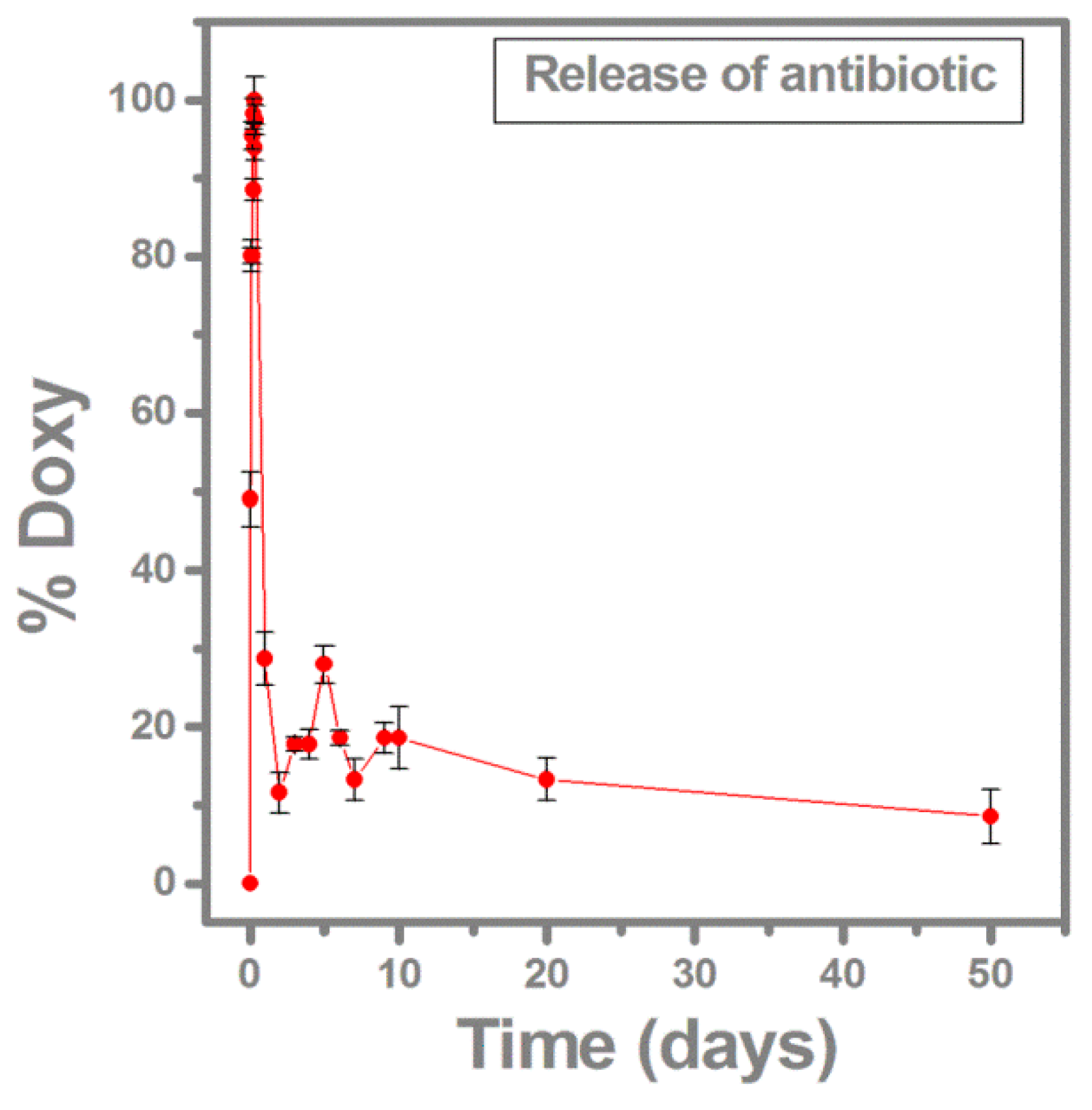
2.2. Antibiotic Release Monitored by FTIR and UV-VIS
2.3. Electrochemical Polarization Measurements
2.4. In Vitro Biocompatibility Assay
2.5. Anti-Biofilm Activity of the Synthesized Thin Films
3. Discussion
4. Materials and Methods
4.1. Materials and Experiment
4.2. Physical–Chemical Characterization of Deposited Thin Films
4.2.1. Morphological Examination
4.2.2. Composition
4.2.3. Electrochemical Investigation
4.3. Biological Assays
4.3.1. Biocompatibility
4.3.2. Antimicrobial Biofilm Activity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MAPLE | Matrix-Assisted Pulsed Laser Evaporation |
| PLD | Pulsed Laser Deposition |
| CHA | Carbonated Hydroxyapatite |
| VCCs | Viable cell counts |
| SS | Stainless steel type 316L plates |
| BG | Bioactive glass |
| PMMA | Poly (methyl methacrylate) |
| Doxy | Doxycycline |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive X-ray Spectroscopy |
| FTIR | Fourier Transformed Infrared Spectroscopy |
| SBF | Simulated Body Fluid |
| LSW | Linear sweep voltammetry |
| Ecorr | Corrosion potential |
| icorr | Corrosion current |
| EIS | Electrochemical impedance spectroscopy |
| MG63 | Human bone osteosarcoma cells |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| PI | Propidium Iodide |
| RPMI | Roswell Park Memorial Institute |
| PBS | Phosphate buffered saline |
| tRNA | Transfer ribonucleic acid |
| TSA | Tryptic soy agar |
References
- Global Implantable Biomaterials Market Outlook (2014–2022). Available online: http://www.reportlinker.com/p03152812-summary/Global-Implantable-Biomaterials-Market-Outlook.html (accessed on 4 April 2016).
- Lebeaux, D.; Ghigo, J.M. Management of biofilm-associated infections: What can we expect from recent research on biofilm lifestyles. Med. Sci. 2012, 28, 727–739. [Google Scholar]
- Costerton, J.W.; Donlan, R.M. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar]
- Grumezescu, A.M. Essential oils and nanotechnology for combating microbial biofilms. Curr. Org. Chem. 2013, 17, 90–96. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lappin-Scott, H.M. Behavior of bacteria in biofilms. ASM News 1989, 55, 650–654. [Google Scholar]
- Lazar, V.; Chifiriuc, C. Chapter in book: Mechanisms and experimental models for the assessment of biofilms phenotypic resistance/tolerance. Sci. Microbial Pathogens Commun. Curr. Res. Technol. Adv. 2011. [Google Scholar]
- Brandt, C.M.; Duffy, C.T.; Berbari, E.F.; Hanssen, A.D.; Steckelberg, J.M.; Osmon, R.D. Staphylococcus aureus prosthetic joint infection treated with prosthesis removal and delayed reimplantation arthroplasty. Mayo Clin. Proc. 1999, 74, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, D.; Haschke, M.; Rajacic, Z.; Zimmerli, W.; Trampuz, A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureusin experimental foreign-body infection. Antimicrob. Agents Chemother. 2009, 53, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, J.C.; Munoz-Mahamud, E.; Vilchez, F.; Garcia-Ramiro, S.; Bori, G.; Sierra, J.; Martinez, J.A.; Font, L.; Mensa, J.; Soriano, A. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob. Agents Chemother. 2009, 53, 4772–4777. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Lee, M.S.; Hsu, K.Y.; Chang, Y.H.; Shih, H.N.; Ueng, S.W. Gram-negative prosthetic joint infections: Risk factors and outcome of treatment. Clin. Infect. Dis. 2009, 49, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Tafin, U.F.; Betrisey, B.; Borens, O.; Trampuz, A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob. Agents Chemother. 2013, 57, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Kucharíková, S.; van Dijck, P.; Erdtmann, M.; Krona, A.; Lövenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S.; Duan, S.; Xuliang, D.; Sun, Y.; Zhang, X.; Xu, X.; Guan, B.; Wang, C.; Hu, M.; et al. Modification of Titanium Substrates with Chimeric Peptides Comprising Antimicrobial and Titanium-Binding Motifs Connected by Linkers to Inhibit Biofilm Formation. ACS Appl. Mater. Interfaces 2016, 8, 5124–5136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ragel, C.V.; Vallet-Regi, M.; Arcos, D. Bioactivity in glass/PMMA composites used as drug delivery system. Biomaterials 2001, 22, 701–708. [Google Scholar]
- Vasilev, K.; Cook, J.; Griesser, J.H. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Woo, G.L.Y.; Yang, M.L.; Yin, H.Q.; Jaffer, F.; Mittelman, M.W.; Santerre, J.P. Biological characterization of a novel biodegradable antimicrobial polymer synthesized with fluoroquinolones. J. Biomed. Mater. Res. Part A 2002, 59, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hae-Won, K.; Knowles, J.C.; Kim, H.E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar]
- Craciun, D.; Socol, G.; Cristea, D.; Floroian, L.; Badea, M.; Socol, M.; Craciun, V.; Popescu-Pelin, G. Investigations of Pulsed Laser Deposited TiN Thin Films for Titanium Implants. Romanian Rep. Phys. 2015, 67, 1491–1502. [Google Scholar]
- Del Curto, B.; Brunella, M.F.; Giordano, C.; Pedeferri, M.P.; Valtulina, V.; Visai, L.; Cigada, A. Decreased bacterial adhesion to surface-treated titanium. Int. J. Artif. Organs 2005, 28, 718–748. [Google Scholar] [PubMed]
- Wang, X.; Qiu, S.; Yao, X.; Tang, T.; Dai, K.; Zhu, Z. Berberine inhibits Staphylococcus epidermidis adhesion and biofilm formation on the surface of titanium alloy. J. Orthop. Res. 2009, 27, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.S.; Alves, N.M.; Mano, J.F. Nanostructured multilayer coatings combining chitosan with bioactive glass nanoparticles. J. Nanosci. Nanotechnol. 2008, 9, 1741–1748. [Google Scholar] [CrossRef]
- Sanpo, N.; Tan, M.L.; Cheang, P.; Khor, K.A. Antibacterial property of cold-sprayed HAAg/PEEK coating. J. Therm. Spray Technol. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Patenge, N.; Arndt, K.; Eggert, T.; Zietz, C.; Kreikemeyer, B.; Bader, R.; Nebe, B.; Stranak, V.; Hippler, R.; Podbielski, A. Evaluation of antimicrobial effects of novel implant materials by testing the prevention of biofilm formation using a simple small scale medium-throughput growth inhibition assay. Biofouling 2012, 28, 267–277. [Google Scholar] [PubMed]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2008, 88, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Erakovic, S.; Ristoscu, C.; Mihailescu, N.; Duta, L.; Visan, A.; Stan, G.E.; Popa, A.C.; Husanu, M.A.; Luculescu, C.R.; et al. Structural and biological evaluation of lignin addition to simple and silver-doped hydroxyapatite thin films synthesized by matrix-assisted pulsed laser evaporation. J. Mater. Sci. Mater. Med. 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Erakovic, S.; Jankovic, A.; Ristoscu, C.; Duta, L.; Serban, N.; Visan, A.; Mihailescu, I.N.; Stan, G.E.; Socol, M.; Iordache, O.; et al. Antifungal activity of Ag: Hydroxyapatite thin films synthesized by pulsed laser deposition on Ti and Ti modified by TiO2 nanotubes substrates. Appl. Surf. Sci. 2014, 293, 37–45. [Google Scholar] [CrossRef]
- Rose, W.E.; Otto, D.P.; Aucamp, M.E.; Miller, Z.; de Villiers, M.M. Prevention of biofilm formation by methacrylate-based copolymer films loaded with rifampin, clarithromycin, doxycycline alone or in combination. Pharm. Res. 2014, 32, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Mihaescu, G.; Lazar, V. Medical Microbiology and Virolog; University of Bucharest Publishing House: Bucharest, Romania, 2011. [Google Scholar]
- Socol, G.; Preda, N.; Socol, M.; Sima, L.; Luculescu, C.R.; Sima, F.; Miroiu, M.; Axente, E.; Visan, A.; Stefan, N.; et al. MAPLE deposition of PLGA micro-and nanoparticles embedded into polymeric coatings. Dig. J. Nanomater. Biostruct. 2013, 8, 621–630. [Google Scholar]
- Jones, J.R.; Ehrenfried, L.M.; Hench, L.L. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials 2006, 27, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Ristoscu, C.; Caiteanu, D.; Petrescu, S. Biocompatibility and bioactivity enhancement of Ce stabilized ZrO2 doped HA coatings by controlled porosity change of Al2O3 substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96B, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Reczyński, W.; Janus, A.M.; Engvall, K.; Socha, R.P.; Kotarba, A. Metal release and formation of surface precipitate at stainless steel grade 316 and Hanks solution interface—Inflammatory response and surface finishing effects. Corros. Sci. 2009, 51, 1157–1162. [Google Scholar] [CrossRef]
- Okazakia, Y.; Gotoh, E. Metal release from stainless steel, Co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros. Sci. 2008, 50, 3429–3438. [Google Scholar] [CrossRef]
- Rondelli, G.; Torricelli, P.; Fini, M.; Giardino, R. In vitro corrosion study by EIS of a nickel-free stainless steel for orthopaedic applications. Biomaterials 2005, 26, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, I.N.; Ristoscu, C.; Bigi, A.; Mayer, I. Advanced biomimetic implants based on nanostructured coatings synthesized by pulsed laser technologies. In Laser-Surface Interactions for New Materials Production Tailoring Structure and Properties; Miotello, A., Ossi, P.M., Eds.; Springer Berlin Heidelberg: New York, NY, USA, 2009; Volume 130, pp. 235–260. [Google Scholar]
- Floroian, L.; Florescu, M.; Munteanu, D.; Badea, M.; Popescu-Pelin, G.; Ristoscu, C.; Sima, F.; Chifiriuc, C.M.; Mihailescu, I.N. A new concept of stainless steel medical implant based upon composite nanostructures coating. Dig. J. Nanomater. Biostruct. 2014, 9, 1555–1568. [Google Scholar]
- Cristescu, R.; Popescu, C.; Socol, G.; Visan, A.; Mihailescu, I.N.; Gittard, S.D.; Millerb, P.R.; Martin, T.N.; Narayan, R.J.; Andronie, A.; et al. Deposition of antibacterial of poly(1,3-bis-(p-carboxyphenoxy propane)-co-(sebacic anhydride)) 20:80/gentamicin sulfate composite coatings by MAPLE. Appl. Surf. Sci. 2011, 257, 5287–5292. [Google Scholar]
- Cristescu, R.; Popescu, C.; Socol, G.; Iordache, I.; Mihailescu, I.N.; Mihaiescu, D.E.; Grumezescu, A.M.; Balan, A.; Stamatin, I.; Chifiriuc, C.; et al. Magnetic core/shell nanoparticle thin films deposited by MAPLE: Investigation by chemical, morphological and in vitro biological assays. Appl. Surf. Sci. 2012, 258, 9250–9255. [Google Scholar] [CrossRef]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci. Mater. Med. 2015, 26, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Films 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Floroian, L.; Florescu, M.; Popescu-Pelin, G.; Ristoscu, C.; Mihailescu, I.N. Synthesis of biomaterial thin films by pulsed laser technologies: Electrochemical evaluation of bioactive glass-based nanocomposites coatings for biomedical applications. Mater. Sci. Eng. C 2012, 32, 1152–1157. [Google Scholar] [CrossRef]
- Floroian, L.; Mihailescu, I.N.; Sima, F.; Stanciu, G.; Savu, B. Evaluation of biocompatibility and bioactivity for polymethyl methacrylate—Bioactive glass nanocomposite films obtained bymatrix assisted pulsed laser evaporation. UPB Sci. Bull. A 2010, 72, 134–148. [Google Scholar]
- Kassab, R.; Yammine, P.; Moussa, D.; Safi, N. Microspheres containing Doxycycline: Properties and in vitro study. Int. J. Drug Deliv. 2013, 5, 264–269. [Google Scholar]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignements for calcium, strontiu m and barium hydroxiapatitesutilising isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.T. The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-lead Growth of Functional Materials; Wiley-Interscience: Hoboken NJ, USA, 2007. [Google Scholar]
- Floroian, L.; Sima, F.; Florescu, M.; Badea, M.; Popescu, A.C.; Serban, N.; Mihailescu, I.N. Double layered nanostructured composite coatings with bioactive silicate glass and polymethylmetacrylate for biomimetic implant applications. J. Electroanal. Chem. 2010, 64, 111–118. [Google Scholar] [CrossRef]
- Bitton, G. Microbiology of Drinking Water Production and Distribution; John Wiley and Sons-Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Martínez, J.L.; Baquero, F. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin. Microbiol. Rev. 2002, 15, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Ozyegin, L.S.; Oktar, F.N.; Goller, G.; Kayali, S.; Yazici, T. Plasma-sprayed bovine hydroxyapatite coatings. Mater. Lett. 2004, 58, 2605–2609. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. (Eds.) Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer Science & Business Media: Berlin, Germany, 2012.
- Fawzy, A.S.; Amer, A.M. An in vitro and in vivo evaluation of bioactive titanium implants following sodium removal treatment. Dent. Mater. 2009, 2, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Padilla, R.J.; Ambrose, W.; De Kok, J.I.; Cooper, L.F. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Wildemann, B.; Schmidmaier, G.; Stockle, U.; Lucke, M. Gentamycin delivered from a PDLLA coating of metallic implants in vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury 2010, 41, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Kessler, B.; Wolf, E.; Jordan, G.; Mohl, G.S. Winter, Correlation of in vivo and in vitro release data for rh-INFa lipid implants. Eur. J. Pharm. Biopharm. 2008, 70, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, C.; Manzano, M.; Vallet-Regí, M. Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomed. 2008, 3, 403–414. [Google Scholar]
- Popa, M.; Hussien, M.D.; Cirstea, A.; Grigore, R.; Lazar, V.; Bezirtzoglou, E.; Chifiriuc, C.; Sakizlian, M.; Stavropoulou, E.; Bertesteanu, S. Insights in Metal Based Dental Implants and Their Interaction with the Surrounding Tissues. Curr. Top. Med. Chem. 2015, 15, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Potapova, I. Functional Imaging in Diagnostic of Orthopedic Implant-Associated Infections. Diagnostics 2013, 3, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive Coatings for Orthopaedic Implants—Recent Trends in Development of Implant Coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Mellefont, L.A.; McMeekin, T.A.; Ross, T. The effect of abrupt osmotic shifts on the lag phase duration of foodborne bacteria. Int. J. Food. Microbiol. 2003, 83, 281–293. [Google Scholar] [CrossRef]
- Mellefont, L.A.; Ross, T. The effect of abrupt shifts in temperature on the lag phase duration of Escherichia coli and Klebsiella oxytoca. Int. J. Food Microbiol. 2003, 83, 295–305. [Google Scholar] [CrossRef]
- Croitoru, S.M.; Mihailescu, I.N.; Maris, D.A.; Popovici, I.A. Experimental research of titanium implants. In Proceedings of the International Working Conference Total Quality Management Advanced and Intelligent Approaches, Belgrade, Serbia, 1 May–4 June 2015.
- Jardini, A.L.; Larosa, M.A.; CarvalhoZavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Kharmandayan, P.; Calderoni, D.; Filho, R.M. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D.J. Synthesis of functionally graded bioactive glass-apatite multistructures on Ti substrates by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, Z.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive glass and hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Geringer, J.; Atmani, F.; Forest, B. Friction–corrosion of AISI 316L/bone cement and AISI 316L/PMMA contacts: Ionic strength effect on tribological behavior. Wear 2009, 267, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Thaker, M.; Spanogiannopoulos, P.; Wright, G.D. The tetracycline resistome. Cell. Mol. Life Sci. 2010, 67, 419–431. [Google Scholar] [CrossRef] [PubMed]
- McGill, R.A.; Chrisey, D.B. Method of Producing a Film Coating by Matrix Assisted Pulsed Laser Deposition. U.S. Patent 6025036 A, 15 February 2000. [Google Scholar]
- Schou, J. Fundamentals of laser-assisted fabrication of inorganic and organic films. In Functionalized Nanoscale Materials, Devices and Systems (NATO Science for Peace and Security Series B: Physics and Biophysics); Mihailescu, I.N., Vaseashta, A., Eds.; Springer Science & Business Media: Dordrecht, Germany, 2008; pp. 241–256. [Google Scholar]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Soprony, M.; Luculescu, C.; Freche, M.; Marsan, O.; et al. Biomimetic nanocrystallineapatitecoatings synthesized by Matrix Assisted Pulsed Laser Evaporation for medical applications. Mater. Sci. Eng. B 2014, 181, 56–63. [Google Scholar] [CrossRef]
- Cristescu, R.; Cojanu, C.; Popescu, A.; Grigorescu, S.; Duta, L.; Caraene, G.; Ionescu, A.; Mihaiescu, D.; Albulescu, R.; Buruiana, T.; et al. Functionalized Polyvinyl Alcohol Derivatives Thin Films for Controlled Drug Release and Targeting Systems: MAPLE Deposition and Morphological, Chemical and in Vitro Characterization. Appl. Surf. Sci. 2009, 255, 5600–5604. [Google Scholar]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3J. J. Biomed. Mater. Res. Part A 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.








| Sample | TIME | icorr (μA/cm2) | Ecorr (mV) |
|---|---|---|---|
| SS | 0 days | 15.38 ± 0.41 | −625.45 ± 0.32 |
| 14 days | 18.22 ± 0.12 | −773.91 ± 0.21 | |
| 28 days | 25.14 ± 0.34 | −997.38 ± 0.22 | |
| BG-PMMA-Doxy/SS | 0 days | 6.21 ± 0.12 | −389.02 ± 0.34 |
| 14 days | 7.15 ± 0.31 | −425.18 ± 0.10 | |
| 28 days | 6.96 ± 0.07 | −420.03 ± 0.12 |
| Sample | Time (Days) | Max Phase Angle (deg) | |
|---|---|---|---|
| SS | 0 | −58 ± 4 | |
| 7 | −55 ± 2 | ||
| 14 | −43 ± 2 | ||
| 21 | −40 ± 4 | ||
| 28 | −36 ± 2 | ||
| BG-PMMA-Doxy/SS | 0 | −67 ± 4 | |
| 7 | −54 ± 4 | ||
| 14 | −43 ± 3 | −24 ± 2 | |
| 21 | −40 ± 3 | −28 ± 2 | |
| 28 | −50 ± 3 | ||
| Ions | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HPO4 2− | SO42− | HCO3− |
|---|---|---|---|---|---|---|---|---|
| Composition (mM) | 142 | 5 | 1.5 | 2.5 | 147.8 | 1 | 0.5 | 4.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules 2016, 21, 740. https://doi.org/10.3390/molecules21060740
Floroian L, Ristoscu C, Mihailescu N, Negut I, Badea M, Ursutiu D, Chifiriuc MC, Urzica I, Dyia HM, Bleotu C, et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules. 2016; 21(6):740. https://doi.org/10.3390/molecules21060740
Chicago/Turabian StyleFloroian, Laura, Carmen Ristoscu, Natalia Mihailescu, Irina Negut, Mihaela Badea, Doru Ursutiu, Mariana Carmen Chifiriuc, Iuliana Urzica, Hussien Mohammed Dyia, Coralia Bleotu, and et al. 2016. "Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings" Molecules 21, no. 6: 740. https://doi.org/10.3390/molecules21060740







