Synthesis of a Conjugated D-A Polymer with Bi(disilanobithiophene) as a New Donor Component
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Optical and Electrochemical Properties of pDSBT2-BT
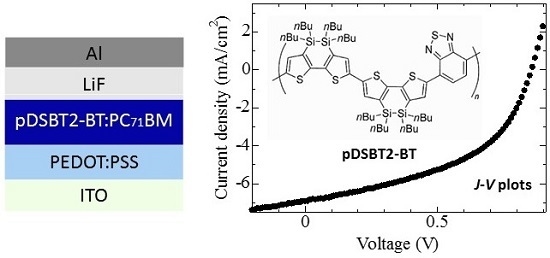
2.3. Device Fabrication
3. Materials and Methods
3.1. General
3.2. Device Fabrication
3.3. Preparation of pDSBT2-BT
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dou, L.; Jingbi, Y.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th Anniversary Article: A Decade of Organic/Polymeric Photovoltaic Research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, H.Y.; Zhang, S.; Li, G.; Yang, Y. Synthesis, characterization, and photovoltaic properties of a low band gap polymer based on silole-containing polythiophenes and 2,1,3-benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J. Conjugated oligomers and polymers containing dithienosilole units. Macromol. Chem. Phys. 2009, 210, 1360–1370. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Pisula, W.; Tsao, H.N.; Ellinger, S.; Müllen, K.; Reynolds, J.R. Tailoring structure-property relationships in dithienosilole-benzothiadiazole donor-acceptor copolymers. J. Am. Chem. Soc. 2009, 131, 7514–7515. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.Y.; Lu, J.P.; Beaupré, S.; Zhang, Y.; Pouliot, J.R.; Zhou, J.; Najari, A.; Leclerc, M.; Tao, Y. Effects of the molecular weight and the side-chain length on the photovoltaic performance of dithienosilole/thienopyrrolodione copolymers. Adv. Funct. Mater. 2012, 22, 2345–2341. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Xin, H.; Kim, F.S.; Shoaee, S.; Durrant, J.R.; Jenekhe, S.A. Effects of side chains on thiazolothiazole-based copolymer semiconductors for high performance solar cells. Adv. Energy Mater. 2011, 1, 854–860. [Google Scholar] [CrossRef]
- Ohshita, J.; Hwang, Y.M.; Mizumo, T.; Yoshida, H.; Ooyama, Y.; Harima, Y.; Kunugi, Y. Synthesis of dithienogermole-containing π-conjugated polymers and applications to photovoltaic cells. Organometallics 2011, 12, 3233–3236. [Google Scholar] [CrossRef]
- Small, C.E.; Chen, S.; Subbiah, J.; Amb, C.M.; Tsang, S.W.; Lai, T.H.; Reynolds, J.R.; So, F. High-efficiency inverted dithienogermole-thienopyrrolodione-based polymer solar cells. Nat. Photonics 2012, 6, 115–120. [Google Scholar] [CrossRef]
- Amb, C.M.; Chen, S.; Graham, K.R.; Subbiah, J.; Samll, C.E.; So, F.; Reynorlds, J.R. Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J. Am. Chem. Soc. 2011, 133, 10062–10065. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Miyazaki, M.; Nakashima, M.; Tanaka, D.; Ooyama, Y.; Sasaki, T.; Kunugi, Y.; Morihara, Y. Synthesis of conjugated D-A polymers bearing bi(dithienogermole) as a new donor component and their applications to polymer solar cells and transistors. RSC Adv. 2015, 5, 12686–12691. [Google Scholar] [CrossRef]
- Ohshita, J.; Nakashima, M.; Tanaka, D.; Morihara, Y.; Fueno, H.; Tanaka, K. Preparation of a D-A polymer with disilanobithiophene as a new donor component and application to high-voltage bulk heterojunction polymer solar cells. Polym. Chem. 2014, 5, 346–349. [Google Scholar] [CrossRef]
- Nakashima, M.; Otsura, T.; Naito, H.; Ohshita, J. Synthesis of new D-A polymers containing disilanobithiophene donor and application to bulk heterojunction polymer solar cells. Polym. J. 2015, 47, 733–738. [Google Scholar] [CrossRef]
- Ohshita, J.; Adachi, Y.; Tanaka, D.; Nakashima, M.; Ooyama, Y. Synthesis of D-A polymers with a disilanobithiophene donor and a pyridine or pyrazine acceptor and their applications to dyesensitized solar cells. RSC Adv. 2015, 5, 36673–36679. [Google Scholar] [CrossRef]
- Sample Availability: Not available.







| Polymer | Mn (Mw/Mn) b/gmol−1 | UV-vis abs λmax/nm | Eg c/eV | EHOMO d/eV | ELUMO e/eV | |
|---|---|---|---|---|---|---|
| Solution | Film | |||||
| pDSBT f | 7200 (2.0) | 542 | nd g | 1.9 | nd g | |
| pDSBT-BT h | 20,000 (1.9) | 633, 680 | 650, 689 | 1.7 | −5.2 | −3.5 |
| pDSBT2-BT | 13,000 (1.9) | 599 | 629, 668 | 1.8 | −5.2 | −3.4 |
| Run | Cell Type a | ETL (Thickness/nm) | Annealing Temp/°C | Additive (/vol %) | Active Layer Thickness/nm | Voc/V | Jsc /mAcm−2 | FF | PCE/% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Con | Ca (5) | non | non | 70 | 0.86 | 6.91 | 0.48 | 2.8 |
| 2 | Con | Ca (25) | non | non | 70 | 0.81 | 4.04 | 0.35 | 1.2 |
| 3 | Con | LiF (0.5) | non | non | 70 | 0.85 | 7.03 | 0.49 | 2.9 |
| 4 | Con | LiF (1.0) | non | non | 70 | 0.85 | 5.63 | 0.40 | 1.9 |
| 5 | Con | LiF (1.5) | non | non | 70 | 0.85 | 6.29 | 0.45 | 2.4 |
| 6 | Con | LiF (0.5) | 50 | non | 70 | 0.85 | 5.57 | 0.43 | 2.0 |
| 7 | Con | LiF (0.5) | 80 | non | 70 | 0.84 | 5.47 | 0.42 | 1.9 |
| 8 | Con | LiF (1.5) | non | DIO b (1) | 70 | 0.52 | 5.14 | 0.40 | 1.1 |
| 9 | Con | LiF (1.5) | non | DIO b (2.5) | 70 | 0.33 | 2.06 | 0.26 | 0.2 |
| 10 | Con | LiF (0.5) | non | non | 60 | 0.86 | 7.56 | 0.51 | 3.3 |
| 11 | Con | LiF (0.5) | non | non | 50 | 0.87 | 7.14 | 0.48 | 3.0 |
| 12 | Con | LiF (0.5) | non | non | 40 | 0.88 | 6.33 | 0.46 | 2.6 |
| 13 | Inv | 0.79 | 5.99 | 0.42 | 2.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, M.; Ooyama, Y.; Sugiyama, T.; Naito, H.; Ohshita, J. Synthesis of a Conjugated D-A Polymer with Bi(disilanobithiophene) as a New Donor Component. Molecules 2016, 21, 789. https://doi.org/10.3390/molecules21060789
Nakashima M, Ooyama Y, Sugiyama T, Naito H, Ohshita J. Synthesis of a Conjugated D-A Polymer with Bi(disilanobithiophene) as a New Donor Component. Molecules. 2016; 21(6):789. https://doi.org/10.3390/molecules21060789
Chicago/Turabian StyleNakashima, Makoto, Yousuke Ooyama, Takuya Sugiyama, Hiroyoshi Naito, and Joji Ohshita. 2016. "Synthesis of a Conjugated D-A Polymer with Bi(disilanobithiophene) as a New Donor Component" Molecules 21, no. 6: 789. https://doi.org/10.3390/molecules21060789
APA StyleNakashima, M., Ooyama, Y., Sugiyama, T., Naito, H., & Ohshita, J. (2016). Synthesis of a Conjugated D-A Polymer with Bi(disilanobithiophene) as a New Donor Component. Molecules, 21(6), 789. https://doi.org/10.3390/molecules21060789