Syk and IRAK1 Contribute to Immunopharmacological Activities of Anthraquinone-2-carboxlic Acid
Abstract
:1. Introduction
2. Results
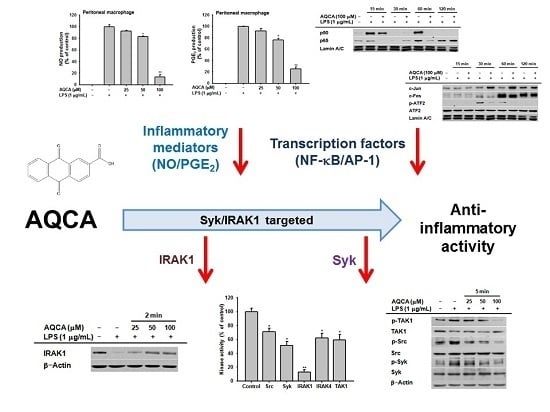
2.1. Effect of AQCA on Inflammatory Responses of Macrophages Induced by LPS
2.2. Effect of AQCA on Transcriptional Activation of the Inflammatory Response
2.3. Effect of AQCA on the Activation of Upstream Enzymes for NF-κB and AP-1 Activation
2.4. Effect of AQCA on the Activity of Upstream Enzymes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Peritoneal Macrophages
4.3. Cell Culture and Drug Preparation
4.4. Determination of Nitric Oxide (NO) and Prostaglandin E2 (PGE2) Production
4.5. Cell Viability Assay
4.6. Preparation of Whole Cell Lysates and Nuclear Fractions for Immunoblot Analyses
4.7. In Vitro Kinase Assay with Purified Enzymes
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NO | nitric oxide |
| MTT | (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, tetrazole |
| AQCA | anthraquinone-2-carboxylic acid |
| 2-HMAQ | 2-hydroxymethylanthraquinone |
| PMA | phorbol-12-myristate |
| COX-2 | cyclooxygenase-2 |
| FBS | fetal bovine serum |
| iNOS | inducible nitric oxide synthase |
| IKK | IκB kinase |
| LPS | lipopolysaccharide |
| PAMPs | pathogen-associated molecular patterns |
| PBS | phosphate-buffered saline |
| PRRs | pattern recognition receptors |
| TLRs | toll-like receptors |
| TNF | tumor necrosis factor |
| Syk | spleen tyrosine kinase |
| IRAK4 | interleukin-1 receptor-associated kinase 4 |
References
- Lim, S.; Park, S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, T.S.; Lee, J.G.; Park, J.K.; Yang, M.; Kim, J.M.; Jo, E.K.; Yuk, J.M. Characterization of proinflammatory responses and innate signaling activation in macrophages infected with Mycobacterium scrofulaceum. Immune Netw. 2014, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Leandro, G.; Mangia, A.; Hui, J.; Fabris, P.; Rubbia–Brandt, L.; Colloredo, G.; Adinolfi, L.E.; Asselah, T.; Jonsson, J.R.; Smedile, A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: A meta-analysis of individual patient data. Gastroenterology 2006, 130, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Toss, H.; Siegbahn, A.; Venge, P.; Wallentin, L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. N. Engl. J. Med. 2000, 343, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Um, M.Y.; Ahn, J.; Jung, C.H.; Park, M.K.; Ha, T.Y. Ethanolic extract of taheebo attenuates increase in body weight and fatty liver in mice fed a high-fat diet. Molecules 2014, 19, 16013–16023. [Google Scholar] [CrossRef] [PubMed]
- Al-Howiriny, T.; Al-Sohaibani, M.; El-Tahir, K.; Rafatullah, S. Prevention of experimentally-induced gastric ulcers in rats by an ethanolic extract of “Parsley” Petroselinum crispum. Am. J. Chin. Med. 2003, 31, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Lee, J.; Kim, J.H.; Yang, W.S.; Kwak, Y.-S.; Kim, S.Y.; Choung, E.S.; Rhee, M.H.; Cho, J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediat. Inflamm. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Kim, S.; Jung, W.J.; Lee, H.S.; Kim, M.Y. Immunomodulatory effects of ZYM-201 on LPS-stimulated B Cells. Immune Netw. 2014, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, Y.S. Korean red ginseng water extract inhibits COX-2 expression by suppressing p38 in acrolein-treated human endothelial cells. J. Ginseng Res. 2014, 38, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Jang, S.; Lim, J.W.; Kang, J.; Bak, E.J.; Cha, J.H.; Kim, H. Protective effect of Korean red ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils. J. Ginseng Res. 2014, 38, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, S.J.; Ahn, E.M.; Oh, S.R.; Lee, H.J.; Jeong, J.A.; Lee, J.Y. Ribes fasciculatum var. chinense attenuated allergic inflammation in vivo and in vitro. Biomol. Ther. 2014, 22, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Chen, H.-L.; Li, H.; Zhang, K.-L.; Chen, X.-Y.; Wang, X.-W.; Kong, Q.-Y.; Liu, J. Regulatory effects of emodin on NF-κB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int. J. Mol. 2005, 16, 41–47. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Aggarwal, B.B. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-κB activation, NF-κB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene 1998, 17, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Kim, S.C.; Kim, Y.H.; Yang, W.S.; Kim, Y.; Hong, S.; Kim, K.H.; Yoo, B.C.; Kim, S.H.; Kim, J.H.; Cho, J.Y. Anti-inflammatory and antinociceptive activities of anthraquinone-2-carboxylic acid. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, L.; Song, B.; Xiao, X.; Qi, M.; Yang, Q.; Yang, Q.; Tang, X.; Wang, Z.; Yang, L. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating SREBP pathway. Eur. J. Pharmacol. 2016, 770, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, M.; Lu, M.; Xi, B.; Sheng, H.; Zang, Y.Q. Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. Am. J. Physiol. Endoc. M. 2011, 300, E886–E893. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Fukaya, Y.; Imai, S.; Yamakuni, T. Beneficial effects of Ajuga decumbens on osteoporosis and arthritis. Biol. Pharm. Bull. 2008, 31, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, Y.J.; Bian, A.H.; Zuo, H.B.; Zhu, T.W.; Ji, S.X.; Kong, F.; Yin, D.Q.; Wang, C.B.; Wang, Z.F. The regulatory role of activating transcription factor 2 in inflammation. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, J.; Rhee, M.H.; Yu, T.; Baek, K.-S.; Sung, N.Y.; Kim, Y.; Yoon, K.; Kim, J.H.; Kwak, Y.-S. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J. Ginseng Res. 2015, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Jeong, H.Y.; Kim, S.H.; Kim, H.G.; Nam, G.; Kim, J.P.; Yoon, D.H.; Hwang, H.; Kimc, T.W.; Hong, S. Methanol extract of Evodia lepta displays Syk/Src-targeted anti-inflammatory activity. J. Ethnopharmacol. 2013, 148, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Lee, Y.G.; Yang, W.S.; Oh, J.; Jeong, D.; Lee, S.; Kim, T.W.; Park, Y.C.; Sung, G.-H. Methanol extract of Hopea odorata suppresses inflammatory responses via the direct inhibition of multiple kinases. J. Ethnopharmacol. 2013, 145, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, J.; Yi, Y.-S.; Yang, Y.; Kim, K.W.; Cho, J.Y. p38/AP-1 pathway in lipopolysaccharide-induced inflammatory responses is negatively modulated by electrical stimulation. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-S.; Son, Y.-J.; Ryou, C.; Sung, G.-H.; Kim, J.-H.; Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Yi, Y.-S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Chain, B.M.; Cho, J.Y. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of κBα via activation of the phosphoinositide-3-kinase and Akt pathways. Int. J. Biochem. Cell Biol. 2009, 41, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S. The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2001, 2, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.G.; Kim, M.-Y.; Byeon, S.E.; Rhee, M.H.; Park, J.; Katz, D.R.; Chain, B.M.; Cho, J.Y. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem. Pharmacol. 2010, 79, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Regulation of innate immunity signaling and its connection with human diseases. Curr. Drug Targets Inflamm. Allergy 2004, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pathria, G.; Garg, B.; Garg, K.; Wagner, C.; Wagner, S.N. Dual JNK-Cyclin D1 and ERK-c-Jun disjunction in human melanoma. Br. J. Dermatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Han, H.S.; Sabapathy, K.; Lee, B.M.; Yu, E.; Choi, J. Expression of a homeostatic regulator, Wip1 (wild-type p53-induced phosphatase), is temporally induced by c-Jun and p53 in response to UV irradiation. J. Biol. Chem. 2010, 285, 9067–9076. [Google Scholar] [CrossRef] [PubMed]
- Joh, E.-H.; Jeong, J.-J.; Kim, D.-H. Inhibitory effect of echinocystic acid on 12-O-tetradecanoylphorbol-13-acetate-induced dermatitis in mice. Arch. Pharm. Res. 2014, 37, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407. [Google Scholar] [CrossRef]
- Kim, M.J.; Kadayat, T.; da Kim, E.; Lee, E.S.; Park, P.H. TI-I-174, a synthetic chalcone derivative, suppresses nitric oxide production in murine macrophages via heme oxygenase-1 induction and inhibition of AP-1. Biomol. Ther. 2014, 22, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Yayeh, T.; Jung, K.-H.; Jeong, H.Y.; Park, J.-H.; Song, Y.-B.; Kwak, Y.-S.; Kang, H.-S.; Cho, J.Y.; Oh, J.-W.; Kim, S.-K. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264. 7 cells and protects mice against endotoxic shock. J. Ginseng Res. 2012, 36, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ahn, H.M.; Shen, T.; Yoon, K.; Jang, H.-J.; Lee, Y.J.; Yang, H.M.; Kim, J.H.; Kim, C.; Han, M.H. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J. Ethnopharmacol. 2011, 137, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, W.S.; Yu, T.; Yi, Y.S.; Park, J.G.; Jeong, D.; Kim, J.H.; Oh, J.S.; Yoon, K.; Cho, J.Y. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem. Pharmacol. 2014, 88, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lee, J.; Lee, Y.G.; Byeon, S.E.; Kim, M.H.; Sohn, E.-H.; Lee, Y.J.; Lee, S.G.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer Tegmentosum. J. Ethnopharmacol. 2010, 128, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Yoo, B.C.; Cho, J.Y. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J. Ginseng Res. 2014, 38, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.S.; Hong, Y.D.; Kim, Y.; Sung, N.Y.; Yang, S.; Lee, K.M.; Park, J.Y.; Park, J.S.; Rho, H.S.; Shin, S.S.; et al. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J. Ginseng Res. 2015, 39, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Lee, M.-Y.; Shin, I.-S.; Seo, C.-S.; Ha, H.; Shin, H.K. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch. Pharm. Res. 2012, 35, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound anthraquinone-2-carboxylic acid is available from the authors.








© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.G.; Son, Y.-J.; Kim, M.-Y.; Cho, J.Y. Syk and IRAK1 Contribute to Immunopharmacological Activities of Anthraquinone-2-carboxlic Acid. Molecules 2016, 21, 809. https://doi.org/10.3390/molecules21060809
Park JG, Son Y-J, Kim M-Y, Cho JY. Syk and IRAK1 Contribute to Immunopharmacological Activities of Anthraquinone-2-carboxlic Acid. Molecules. 2016; 21(6):809. https://doi.org/10.3390/molecules21060809
Chicago/Turabian StylePark, Jae Gwang, Young-Jin Son, Mi-Yeon Kim, and Jae Youl Cho. 2016. "Syk and IRAK1 Contribute to Immunopharmacological Activities of Anthraquinone-2-carboxlic Acid" Molecules 21, no. 6: 809. https://doi.org/10.3390/molecules21060809