Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics
Abstract
:1. Introduction
2. Results and Discussion
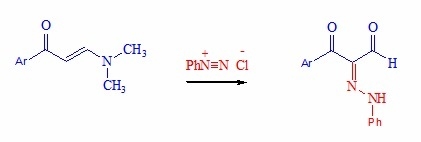
2.1. Chemistry
2.2. Effect of Concentration of Carrier
2.3. Effect of Dyeing Temperature
3. Materials and Methods
3.1. General Procedure for the Synthesis of Disperse Dyes (3a,b)
3.2. Fabrics
3.3. Dyeing Process
3.4. Color Measurements
3.5. Fastness Properties
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Reigel, E.R.; Kent, J.A. Riegel’s Handbook of Industrial Chemsitry, 10th ed.; Springer Pub: New York, NY, USA, 2003; pp. 896–904. [Google Scholar]
- Broadbent, A.D. Basic Principles of Textile Coloration, Society of Dyers and Colorists; Thanet Press Ltd: Kent, West Yorkshire, England, 2001; pp. 322–331. [Google Scholar]
- Lai, C.C.; Chen, K.M. Dyeing properties of modified gemini surfactants on a disperse dye-polyester system. Text. Res. J. 2008, 78, 382–389. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, C.; Tanga, R.C.; Chen, G. Study on the dyeing properties of hemicyanine dyes. II. Cationic dyeable polyester. J. Soc. Dyers Colour. 2012, 128, 147–152. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; Al-Awadi, N.A.; El-Apasery, M.A.; Ibrahim, M.R. Synthesis of some novel pyrazolo[1,5-a]pyrimidine derivatives and their application as disperse dyes. Molecules 2011, 16, 5182–5193. [Google Scholar] [CrossRef] [PubMed]
- El-Apasery, M.A. Solvent-free one-pot synthesis of some azodisperse dyes under microwave irradiation: Dyeing of polyester fabrics. J. Appl. Polym. Sci. 2008, 109, 695–699. [Google Scholar] [CrossRef]
- El-Apasery, M.A. Synthesis of some azo dispersedyes by the use of focused microwave heating. Pol. J. Appl. Chem. 2006, 50, 75–81. [Google Scholar]
- Al-Etaibi, A.; El-Apasery, M.A.; Kamel, M.M. Dyeing of polyester with disperse dyes: Part 1. Antimicrobial activity and dyeing performance of some disperse dyes. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 923–928. [Google Scholar]
- Sadov, F.; Korchagin, M.; Matetsky, A. Chemical Technology of Fibrous Materials, 1st ed.; Mir Publishers: Moscow, Russia, 1973; pp. 332–336. [Google Scholar]
- Mashaly, H.M.; Abdelghaffar, R.A.; Kamel, M.M.; and Youssef, B.M. Dyeing of Polyester Fabric using Nano Disperse Dyes and Improving Their Light Fastness using ZnO Nano Powder. Ind. J. Sci. Technol. 2014, 7, 960–967. [Google Scholar]
- Ding, M.; Ma, S.; Liu, D. Simultaneous Determination of Hydroxyanthraquinones in Rhubard and Experimental Animal Bodies by High-Performance Liquid Chromatography. Anal. Sci. 2003, 19, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kumari, S.; Gulrajani, M. Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 2: Dyeing of nylon and polyester with nordamncanthal. Color. Technol. 2001, 117, 333–336. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A.; Huda, H.M.; Al-Awadi, N.A. One-pot synthesis of disperse dyes under microwave irradiation: Dyebath reuse in dyeing of polyester fabrics. Molecules 2012, 17, 4266–4280. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.F.; Ahmed, R.M.; Marie, M.M. Optimizing the Dyeing Process of Alkali-Treated Polyester Fabric with Dolu Natural Dye. Int. J. Eng. Res. Appl. 2014, 4, 35–40. [Google Scholar]
- El-Adasy, A.A.A.M.; Kamel, M.K.; Saleh, M.O.; Hussein, A.M.; El-Apasery, M.A. Disperse Dyes Based on Pyrazolopyrimidinones I: Their Dyeing Applications and Antimicrobial Activities. Int. J. ChemTech Res. 2016, 9, 31–38. [Google Scholar]
- Sample Availability: Samples of the compounds 3a,b are available from the authors.



| Dyeing Temp. | % Carrier | K/S | L* | a* | b* | C* | h* | ∆E |
|---|---|---|---|---|---|---|---|---|
| 70 °C | 0 | 0.93 | 87.83 | −3.14 | 4.06 | 5.13 | 127.57 | 87.98 |
| 1 | 1.17 | 87.91 | −4.66 | 8.02 | 9.28 | 120.16 | 88.40 | |
| 2 | 1.19 | 87.25 | −5.73 | 11.47 | 12.82 | 116.56 | 88.19 | |
| 3 | 2.60 | 86.26 | −8.18 | 17.57 | 19.36 | 114.85 | 88.40 | |
| 4 | 1.68 | 87.47 | −7.48 | 16.44 | 18.06 | 114.45 | 89.32 | |
| 5 | 1.92 | 87.51 | −8.59 | 20.05 | 21.81 | 113.20 | 90.19 | |
| 90 °C | 0 | 6.53 | 84.86 | −12.67 | 39.74 | 41.71 | 107.68 | 94.56 |
| 1 | 7.65 | 85.08 | −13.08 | 41.58 | 43.59 | 107.46 | 95.60 | |
| 2 | 8.32 | 84.98 | −13.72 | 43.31 | 45.43 | 107.58 | 96.36 | |
| 3 | 8.90 | 84.79 | −13.42 | 54.47 | 47.04 | 106.44 | 97.14 | |
| 4 | 7.30 | 85.31 | −13.48 | 39.82 | 42.04 | 108.70 | 95.10 | |
| 5 | 6.37 | 85.33 | −12.95 | 83.59 | 40.70 | 108.55 | 94.54 | |
| 100 °C | 0 | 6.34 | 84.82 | −12.70 | 83.64 | 40.68 | 108.19 | 94.07 |
| 1 | 6.80 | 84.83 | −13.26 | 41.02 | 43.11 | 106.91 | 95.16 | |
| 2 | 7.72 | 84.95 | −13.65 | 43.28 | 45.38 | 107.51 | 96.31 | |
| 3 | 9.70 | 84.82 | −14.11 | 48.32 | 50.34 | 106.27 | 98.63 | |
| 4 | 12.21 | 84.81 | −14.26 | 52.16 | 54.08 | 105.29 | 99.58 | |
| 5 | 10.12 | 84.75 | −14.05 | 48.40 | 50.39 | 106.19 | 98.44 |
| Dyeing Temp. | % Carrier | K/S | L* | a* | b* | C* | h* | ∆E |
|---|---|---|---|---|---|---|---|---|
| 70 °C | 0 | 1.69 | 83.92 | −1.84 | 16.74 | 16.85 | 96.28 | 23.29 |
| 1 | 1.85 | 83.08 | −1.40 | 22.01 | 22.06 | 93.64 | 28.54 | |
| 2 | 1.87 | 84.37 | −1.52 | 12.80 | 12.89 | 96.76 | 19.33 | |
| 3 | 2.13 | 82.61 | −1.08 | 26.28 | 26.30 | 92.35 | 32.78 | |
| 4 | 2.04 | 83.25 | −2.83 | 24.52 | 24.68 | 96.59 | 31.13 | |
| 5 | 2.10 | 82.83 | −2.86 | 27.35 | 27.50 | 95.98 | 33.97 | |
| 90 °C | 0 | 3.34 | 80.37 | −0.67 | 39.36 | 39.38 | 90.98 | 46.00 |
| 1 | 3.47 | 80.77 | −1.64 | 40.94 | 40.97 | 92.29 | 47.55 | |
| 2 | 3.90 | 81.26 | −3.19 | 42.35 | 42.47 | 94.30 | 48.99 | |
| 3 | 5.15 | 80.43 | −1.13 | 43.50 | 43.51 | 91.84 | 50.10 | |
| 4 | 4.73 | 81.33 | −2.42 | 41.72 | 41.79 | 93.32 | 48.29 | |
| 5 | 5.12 | 80.06 | −2.07 | 43.69 | 43.47 | 92.71 | 50.40 | |
| 100 °C | 0 | 3.93 | 79.81 | −0.19 | 41.51 | 41.52 | 90.26 | 48.20 |
| 1 | 4.49 | 80.23 | −1.27 | 44.49 | 44.50 | 91.64 | 51.11 | |
| 2 | 5.85 | 78.46 | −1.12 | 49.55 | 49.57 | 88.71 | 56.34 | |
| 3 | 5.92 | 79.28 | 0.11 | 46.04 | 46.06 | 89.86 | 52.75 | |
| 4 | 8.97 | 78.08 | −0.21 | 51.20 | 51.21 | 90.23 | 58.04 | |
| 5 | 7.71 | 77.87 | 2.34 | 50.33 | 50.38 | 87.34 | 57.21 |
| Dye No | Washing Fastness | Rubbing Fastness | Perspiration Fastness | Light Fastness | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline | Acidic | |||||||||||
| Alt | SC | SW | Dry | Wet | Alt | SC | SW | Alt | SC | SW | ||
| 3a | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 |
| 3b | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3–4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Etaibi, A.M.; Alnassar, H.S.; El-Apasery, M.A. Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics. Molecules 2016, 21, 855. https://doi.org/10.3390/molecules21070855
Al-Etaibi AM, Alnassar HS, El-Apasery MA. Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics. Molecules. 2016; 21(7):855. https://doi.org/10.3390/molecules21070855
Chicago/Turabian StyleAl-Etaibi, Alya M., Huda S. Alnassar, and Morsy Ahmed El-Apasery. 2016. "Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics" Molecules 21, no. 7: 855. https://doi.org/10.3390/molecules21070855
APA StyleAl-Etaibi, A. M., Alnassar, H. S., & El-Apasery, M. A. (2016). Dyeing of Polyester with Disperse Dyes: Part 2. Synthesis and Dyeing Characteristics of Some Azo Disperse Dyes for Polyester Fabrics. Molecules, 21(7), 855. https://doi.org/10.3390/molecules21070855