18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. 18α-GA-Induced Cytotoxic Effects on Human Leukemia HL-60 Cells
2.2. 18α-GA Induced Nuclear Condensation, DNA Damage and Fragmentation in HL-60 Cells
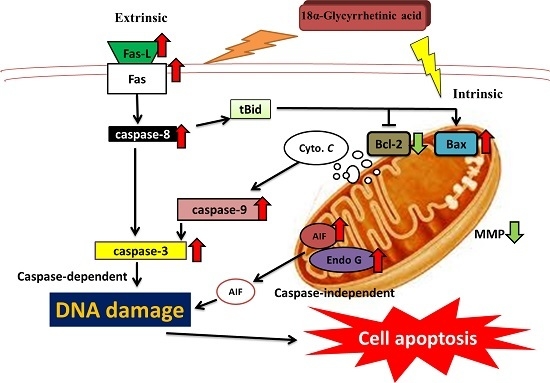
2.3. 18α-GA Decreased the Levels of Mitochondrial Membrane Potential (ΔΨm) and Increased the Activities of Caspase-8, -9 and -3 in HL-60 Cells
2.4. 18α-GA Altered Apoptosis Associated Protein Expression in HL-60 Cells
2.5. 18α-GA Altered the Translocation of Apoptotic Associated Proteins in HL-60 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Proliferation Examination
4.4. Nuclear Staining with DAPI
4.5. Comet Assay
4.6. DNA Gel Electrophoresis
4.7. Measurement of the Levels of Mitochondrial Membrane Potential (ΔΨm)
4.8. Measurements of Caspase-3, Caspase-8 and Caspase-9 Activities
4.9. Western Blotting Analysis
4.10. Confocal Laser Scanning Microscopy Assay
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, H.; Fares, M.; Maguire, K.R.; Siden, A.; Potacova, Z. Cytotoxic effects of tetracycline analogues (doxycycline, minocycline and COL-3) in acute myeloid leukemia HL-60 cells. PLoS ONE 2014, 9, e114457. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program website. Available online: http://seer.cancer.gov/statfacts/html/leuks.html (accessed on 19 May 2014).
- Ministry of Health and Welfare. Caues of death in Taiwan, 2012. Available online: http://www.mohw.gov.tw/CHT/DOS/Statistic.aspx?f_list_no=474&fod_list_no=5007 (accessed on 27 July 2013).
- Bene, M.C.; Nebe, T.; Bettelheim, P.; Buldini, B.; Bumbea, H.; Kern, W.; Lacombe, F.; Lemez, P.; Marinov, I.; Matutes, E.; et al. Immunophenotyping of acute leukemia and lymphoproliferative disorders: A consensus proposal of the European LeukemiaNet Work Package 10. Leukemia 2011, 25, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Robison, L.L. Look, A.T.Acute lymphoblastic leukaemia. Lancet 2008, 371, 1030–1043. [Google Scholar] [CrossRef]
- Warkentin, A.A.; Lopez, M.S.; Lasater, E.A.; Lin, K.; He, B.L.; Leung, A.Y.; Smith, C.C.; Shah, N.P.; Shokat, K.M. Overcoming myelosuppression due to synthetic lethal toxicity for FLT3-targeted acute myeloid leukemia therapy. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 2005, 103, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Philchenkov, A.; Zavelevich, M.; Kroczak, T.J.; Los, M. Caspases and cancer: Mechanisms of inactivation and new treatment modalities. Exp. Oncol. 2004, 26, 82–97. [Google Scholar] [PubMed]
- Jayaraj, R.; Gupta, N.; Rao, P.V. Multiple signal transduction pathways in okadaic acid induced apoptosis in HeLa cells. Toxicology 2009, 256, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Pietraforte, D.; Matarrese, P.; Straface, E.; Gambardella, L.; Metere, A.; Scorza, G.; Leto, T.L.; Malorni, W.; Minetti, M. Two different pathways are involved in peroxynitrite-induced senescence and apoptosis of human erythrocytes. Free Radic. Biol. Med. 2007, 42, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Targeting extrinsic apoptosis in cancer: Challenges and opportunities. Semin. Cell Dev. Biol. 2015, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Y.; Jin, J.; Lang, L.; Zhu, Z.; Fang, W.; Chen, X. Lx2–32c, a novel taxane derivative, exerts anti-resistance activity by initiating intrinsic apoptosis pathway in vitro and inhibits the growth of resistant tumor in vivo. Biol. Pharm. Bull. 2012, 35, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Pde, S.; Ornellas, A.A.; Campos, M.M.; Scheiner, M.A.; Fiedler, W.; Alves, G. Bax and bcl-2 imbalance and HPB infection in penile tumors and adjacent tissues. Prog. Urol. 2004, 14, 353–359. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; You, H.J.; Park, S.J.; Moon, A.R.; Chung, Y.C.; Kang, S.K.; Chun, H.K. Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharmacol. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef]
- Sharma, G.; Kar, S.; Palit, S.; Das, P.K. 18beta-glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J. Cell. Physiol. 2012, 227, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, J.; Mao, C.; Jin, M.; Wu, Q.; Zou, J.; Gu, Q.; Zhang, Y.; Zhang, Y. 18Beta-glycyrrhetinic acid ameliorates acute Propionibacterium acnes-induced liver injury through inhibition of macrophage inflammatory protein-1alpha. J. Biol. Chem. 2010, 285, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, R.G.; Dilshara, M.G.; Park, S.R.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kim, G.Y. 18beta-Glycyrrhetinic acid suppresses TNF-αinduced matrix metalloproteinase-9 and vascular endothelial growth factor by suppressing the AKT-dependent NF-κB pathway. Toxicol. In Vitro 2014, 28, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhong, W.; Li, J.; Zhang, B.; Song, G.; Hu, T. Involvement of BID translocation in glycyrrhetinic acid and 11-deoxy glycyrrhetinic acid-induced attenuation of gastric cancer growth. Nutr. Cancer 2014, 66, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.K.; Iqbal, M.; Athar, M. Inhibitory effect of 18β-glycyrrhetinic acid on 12-O-tetradecanoyl phorbol-13-acetate-induced cutaneous oxidative stress and tumor promotion in mice. Redox Rep. 2005, 10, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J., Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Matsumoto, H.; Sonoda, Y.; Ando, K.; Aizu-Yokota, E.; Sato, T.; Kasahara, T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int. Immunopharmacol. 2004, 4, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Qu, Y.; Xu, M.Y.; Dong, Y.W.; Lu, L.G. 18alpha-glycyrrhetinic acid extracted from Glycyrrhiza radix inhibits proliferation and promotes apoptosis of the hepatic stellate cell line. J. Dig. Dis. 2013, 14, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, V.; Brunati, A.M.; Fiore, C.; Rossi, C.A.; Salvi, M.; Tibaldi, E.; Palermo, M.; Armanini, D.; Toninello, A. Glycyrrhetinic acid as inhibitor or amplifier of permeability transition in rat heart mitochondria. Biochim. Biophys. Acta 2008, 1778, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Hiratsuka, K.; Arikawa, K.; Ono, M.; Komiya, M.; Akimoto, Y.; Fujii, A.; Matsumoto, H. Possible pharmacotherapy for nifedipine-induced gingival overgrowth: 18alpha-glycyrrhetinic acid inhibits human gingival fibroblast growth. Br. J. Pharmacol. 2016, 173, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Karbowiak, I.; Funder, J.W. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin. Endocrinol. 1983, 19, 609–612. [Google Scholar] [CrossRef]
- Armanini, D.; Wehling, M.; Weber, P.C. Mineralocorticoid effector mechanism of liquorice derivatives in human mononuclear leukocytes. J. Endocrinol. Investig. 1989, 12, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Nacamulli, D.; Francini-Pesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids 2005, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.; Shafifiyaz, N.; Hadi, A.H.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Farombi, E.O. Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic Biochem. Physiol. 2015, 118, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tor, Y.S.; Yazan, L.S.; Foo, J.B.; Wibowo, A.; Ismail, N.; Cheah, Y.K.; Abdullah, R.; Ismail, M.; Ismail, I.S.; Yeap, S.K. Induction of Apoptosis in MCF-7 Cells via Oxidative Stress Generation, Mitochondria-Dependent and Caspase-Independent Pathway by Ethyl Acetate Extract of Dillenia suffruticosa and Its Chemical Profile. PLoS ONE 2015, 10, e0127441. [Google Scholar] [CrossRef] [PubMed]
- Minko, T.; Kopeckova, P.; Kopecek, J. Preliminary evaluation of caspases-dependent apoptosis signaling pathways of free and HPMA copolymer-bound doxorubicin in human ovarian carcinoma cells. J. Control. Release 2001, 71, 227–237. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Reed, J.C. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med. 2001, 7, 314–319. [Google Scholar] [CrossRef]
- Santulli, G.; Totary-Jain, H. Tailoring mTOR-based therapy: Molecular evidence and clinical challenges. Pharmacogenomics 2013, 14, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.S.; Shih, Y.L.; Lee, C.H.; Hsueh, S.C.; Liu, J.Y.; Liao, N.C.; Chen, Y.L.; Huang, Y.P.; Lu, H.F.; Chung, J.G. Sulforaphane-induced apoptosis in human leukemia HL-60 cells through extrinsic and intrinsic signal pathways and altering associated genes expression assayed by cDNA microarray. Environ. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Velmurugan, B.K.; Kuo, W.-W.; Chen, Y.-S.; Ho, T.-J.; Tsai, C.-T.; Ye, C.-X.; Tsai, C.-H.; Tsai, F.-J.; Huang, C.-Y. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. Biomedicine 2013, 3, 148–152. [Google Scholar] [CrossRef]
- Patathananone, S.; Thammasirirak, S.; Daduang, J.; Chung, J.G.; Temsiripong, Y.; Daduang, S. Bioactive compounds from crocodile (Crocodylus siamensis) white blood cells induced apoptotic cell death in hela cells. Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-Y.; Lo, H.-Y.; Li, C.-C.; Chen, J.-C.; Hsiang, C.-Y. In vitro and in vivo bioluminescent imaging to evaluate anti-Escherichia coli activity of Galla Chinensis. Biomedicine 2013, 3, 160–166. [Google Scholar] [CrossRef]
- Yu, C.C.; Yang, S.T.; Huang, W.W.; Peng, S.F.; Huang, A.C.; Tang, N.Y.; Liu, H.C.; Yang, M.D.; Lai, K.C.; Chung, J.G. Bisdemethoxycurcumin induces DNA damage and inhibits DNA repair associated protein expressions in NCI-H460 human lung cancer cells. Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Huang, A.C.; Yu, C.S.; Lien, J.C.; Wu, S.H.; Huang, Y.P.; Huang, H.Y.; Kuo, J.H.; Liao, W.Y.; Yang, J.S.; et al. Ellagic acid induces apoptosis in TSGH8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ. Toxicol. 2014, 29, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.







© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Kuo, C.-L.; Lu, K.-W.; Lin, J.-J.; Yang, J.-L.; Wu, R.S.-C.; Wu, P.-P.; Chung, J.-G. 18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways. Molecules 2016, 21, 872. https://doi.org/10.3390/molecules21070872
Huang Y-C, Kuo C-L, Lu K-W, Lin J-J, Yang J-L, Wu RS-C, Wu P-P, Chung J-G. 18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways. Molecules. 2016; 21(7):872. https://doi.org/10.3390/molecules21070872
Chicago/Turabian StyleHuang, Yi-Chang, Chao-Lin Kuo, Kung-Wen Lu, Jen-Jyh Lin, Jiun-Long Yang, Rick Sai-Chuen Wu, Ping-Ping Wu, and Jing-Gung Chung. 2016. "18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways" Molecules 21, no. 7: 872. https://doi.org/10.3390/molecules21070872