Synthesis and Characterization of Magnetic Molecularly Imprinted Polymer for the Enrichment of Ofloxacin Enantiomers in Fish Samples
Abstract
:1. Introduction
2. Results and Discussion
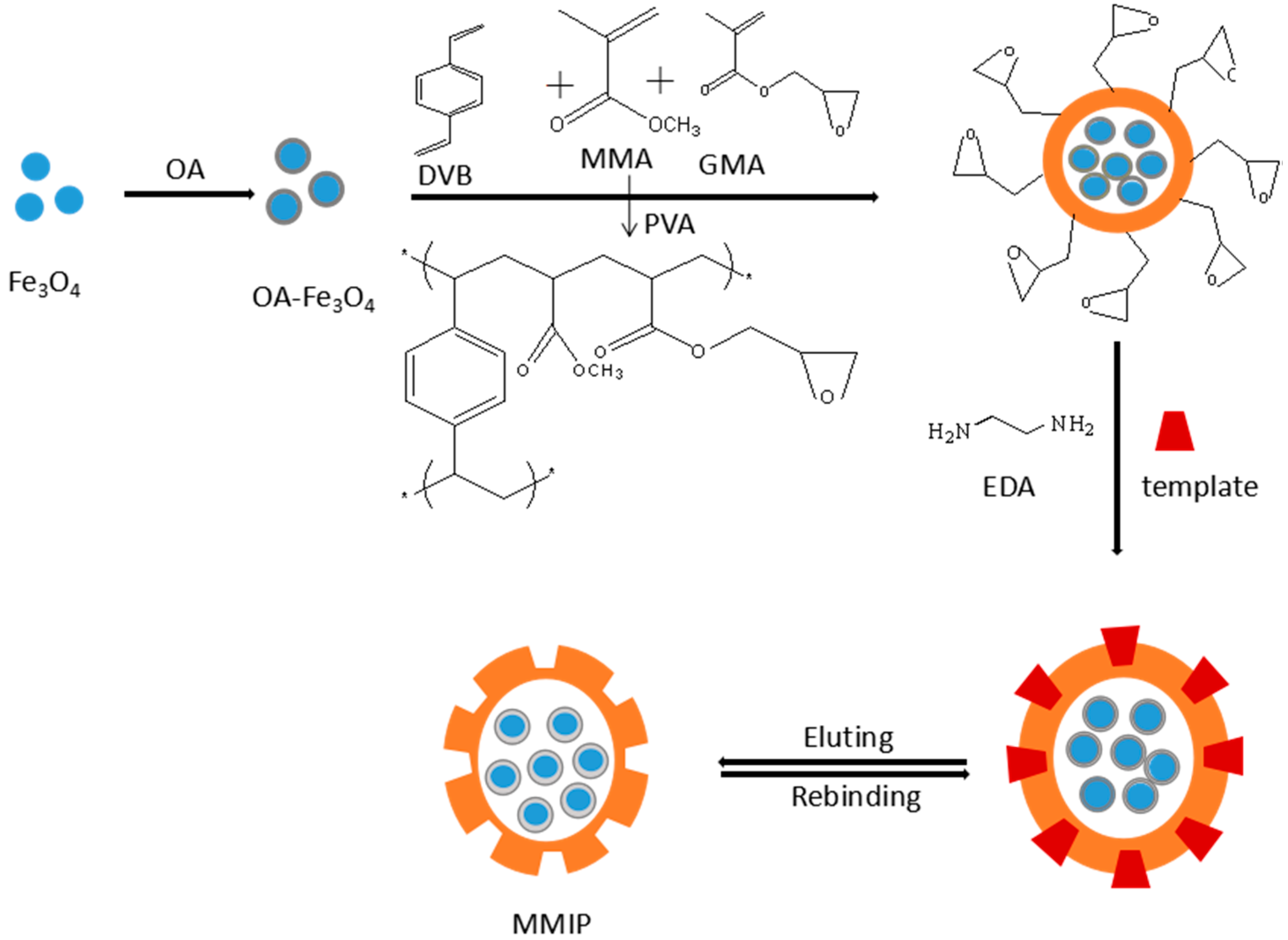
2.1. Synthesis and Characterization of MMIPs
2.2. Adsorption Isotherms
2.3. Optimization of MSPE Conditions
2.3.1. Effect of pH Value
2.3.2. Adsorption Time
2.3.3. Desorption Conditions
2.4. Reusability of MMIPs
2.5. Imprinting Effects of MMIPs and MNIPs on OFL Adsorption
2.6. Application of MMIPs to Biomatrix Samples
3. Materials and Methods
3.1. Materials
3.2. Chromatographic Conditions
3.3. Preparation of Rac-Ofloxacin MMIPs
3.4. Characterization
3.5. Adsorption Studies
3.6. Imprinting Effects of MMIPs and MNIPs on OFL Adsorption
3.7. Separation Enrichment and Determination of OFL Enantiomers in Fish Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixit, S.; Bhushan, R. Chromatographic analysis of chiral drugs. In TLC in Drug Analysis; Komsta, L., Waksmundzka-Hajnos, M., Sherma, J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2014; pp. 97–130. [Google Scholar]
- Shen, Q.; Wang, L.; Zhou, H.; Yu, L.-S.; Zeng, S. Stereoselective binding of chiral drugs to plasma proteins. Acta Pharmacol. Sin. 2013, 34, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Rocco, A.; Fanali, S. Enantiomeric separation of ofloxacin by nano-liquid chromatography using a sulfated-β-cyclodextrin as a chiral selector in the mobile phase. Curr. Anal. Chem. 2010, 6, 209–216. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.-L.; Ma, J.-Q.; Wang, Y.-L. Preparation of ofloxacin poly (glycidyl methacrylate-co-ethylenedimethacrylate)(PGMA/EDMA) molecularly imprinted microspheres and their application to the analysis of quinolones in milk. Food Anal. Methods 2014, 7, 721–729. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Lísa, M.; Holčapek, M. Characterization of triacylglycerol enantiomers using chiral HPLC/APCI-MS and synthesis of enantiomeric triacylglycerols. Anal. Chem. 2013, 85, 1852–1859. [Google Scholar]
- Dong, F.; Chen, X.; Xu, J.; Liu, X.; Chen, Z.; Li, Y.; Zhang, H.; Zheng, Y. Enantioseparation and determination of the chiral fungicide furametpyr enantiomers in rice, soil, and water by high-performance liquid chromatography. Chirality 2013, 25, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lv, X.T.; Zhu, W.X.; Qu, H.Y.; Gao, Y.X.; Guo, B.Y.; Wang, H.L. Enantioselective bioaccumulation of diniconazole in Tenebrio molitor larvae. Chirality 2013, 25, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.K.; Hu, D.Y.; Zhu, H.J.; Yang, J.C.; Song, B.A. Enantioselective degradation of dufulin in four types of soil. J. Agric. Food Chem. 2014, 62, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Kneisel, S.; Auwärter, V. Analysis of 30 synthetic cannabinoids in serum by liquid chromatography-electrospray ionization tandem mass spectrometry after liquid-liquid extraction. J. Mass Spectrom. 2012, 47, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C. Recent Advances in Liquid-Liquid Extraction; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ali, I.; Alam, S.D.; Al-Othman, Z.A.; Farooqi, J.A. Recent advances in SPE? Chiral-HPLC methods for enantiomeric separation of chiral drugs in biological samples. J. Chromatogr. Sci. 2013, 51, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Asgharinezhad, A.A.; Mollazadeh, N.; Ebrahimzadeh, H.; Mirbabaei, F.; Shekari, N. Magnetic nanoparticles based dispersive micro-solid-phase extraction as a novel technique for coextraction of acidic and basic drugs from biological fluids and waste water. J. Chromatogr. A 2014, 1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Z. Preparation of micropipette tip-based molecularly imprinted monolith for selective micro-solid phase extraction of berberine in plasma and urine samples. Talanta 2013, 103, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Asman, S.; Yusof, N.A.; Abdullah, A.H.; Haron, M.J. Synthesis and characterization of hybrid molecularly imprinted polymer (MIP) membranes for removal of methylene blue (MB). Molecules 2012, 17, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Dana, M.; Luliński, P.; Maciejewska, D. Synthesis of Homoveratric Acid-Imprinted Polymers and Their Evaluation as Selective Separation Materials. Molecules 2011, 16, 3826–3844. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, W.; Bao, T.; Chen, Z. Novel molecularly imprinted magnetic nanoparticles for the selective extraction of protoberberine alkaloids in herbs and rat plasma. J. Sep. Sci. 2015, 38, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Azodi-Deilami, S.; Abdouss, M.; Asadi, E.; Najafabadi, A.H.; Sadeghi, S.; Farzaneh, S.; Asadi, S. Magnetic Molecularly Imprinted Polymer Nanoparticles Coupled with High Performance Liquid Chromatography for Solid-Phase Extraction of Carvedilol in Serum Samples. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chen, L.; Lee, Y.K.; Manmana, Y.; Tay, K.S.; Lee, V.S.; Rahman, N.A. Synthesis, characterization, and theoretical study of an acrylamide-based magnetic molecularly imprinted polymer for the recognition of sulfonamide drugs. E-Polymers 2015, 15, 141–150. [Google Scholar] [CrossRef]
- Xiao, D.L.; Dramou, P.; Xiong, N.Q.; He, H.; Li, H.; Yuan, D.H.; Dai, H. Development of novel molecularly imprinted magnetic solid-phase extraction materials based on magnetic carbon nanotubes and their application for the determination of gatifloxacin in serum samples coupled with high performance liquid chromatography. J. Chromatogr. A 2013, 1274, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Azodi-Deilami, S.; Najafabadi, A.H.; Asadi, E.; Abdouss, M.; Kordestani, D. Magnetic molecularly imprinted polymer nanoparticles for the solid-phase extraction of paracetamol from plasma samples, followed its determination by HPLC. Microchim. Acta 2014, 181, 1823–1832. [Google Scholar] [CrossRef]
- Gao, L.; Chen, L.G.; Li, X.W. Magnetic molecularly imprinted polymers based on carbon nanotubes for extraction of carbamates. Microchim. Acta 2015, 182, 781–787. [Google Scholar] [CrossRef]
- Ma, R.T.; Shi, Y.P. Magnetic molecularly imprinted polymer for the selective extraction of quercetagetin from Calendula officinalis extract. Talanta 2015, 134, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, F.; Mullot, J.U.; Pichon, V.; Tuffal, G.; Hennion, M.C. Molecularly imprinted polymers for the clean-up of a basic drug from environmental and biological samples. J. Chromatogr. A 2006, 1135, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Halhalli, M.R.; Sellergren, B. Insights into the formation, structural properties and performance of RAFT polymerized l-phenylalanine anilide molecularly imprinted polymers. Polym. Chem. 2015, 6, 7320–7332. [Google Scholar] [CrossRef]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of chirality and macroring in imprinted polymers with enantiodiscriminative power. ACS Appl. Mater. Int. 2015, 7, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, C.; Dai, H.; Peng, J.; He, J.; Zhang, K.; Kong, S.; Qiu, P.; He, H. Applications of magnetic surface imprinted materials for solid phase extraction of levofloxacin in serum samples. J. Mol. Recognit. 2015, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, Y.G.; Gao, X.F.; OuYang, X.K.; Yang, L.Y.; Yu, D.; Wu, W.J.; Xu, H.P. Pharmacokinetic study of ofloxacin enantiomers in Pagrosomus major by chiral HPLC. Biomed. Chromatogr. 2015. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Linh, P.; Manh, D.; Phong, P.; Hong, L.; Phuc, N. Magnetic properties of Fe3O4 nanoparticles synthesized by coprecipitation method. J. Supercond. Novel Magn. 2014, 27, 2111–2115. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhou, L.X.; Pan, S.D.; Zhan, P.P.; Chen, X.H.; Jin, M.C. Fast determination of 22 sulfonamides from chicken breast muscle using core-shell nanoring amino-functionalized superparamagnetic molecularly imprinted polymer followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1345, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-D.; Shen, H.-Y.; Zhou, L.-X.; Chen, X.-H.; Zhao, Y.-G.; Cai, M.-Q.; Jin, M.-C. Controlled synthesis of pentachlorophenol-imprinted polymers on the surface of magnetic graphene oxide for highly selective adsorption. J. Mater. Chem. A 2014, 2, 15345–15356. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhao, Y.G.; Shen, H.Y.; Zhou, L.X.; Pan, S.D.; Jin, M.C. Fast determination of seven synthetic pigments from wine and soft drinks using magnetic dispersive solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1346, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-G.; Chen, X.-H.; Pan, S.-D.; Zhu, H.; Shen, H.-Y.; Jin, M.-C. Self-assembly of a surface bisphenol A-imprinted core-shell nanoring amino-functionalized superparamagnetic polymer. J. Mater. Chem. A 2013, 1, 11648–11658. [Google Scholar] [CrossRef]
- Xie, X.; Chen, L.; Pan, X.; Wang, S. Synthesis of magnetic molecularly imprinted polymers by reversible addition fragmentation chain transfer strategy and its application in the Sudan dyes residue analysis. J. Chromatogr. A 2015, 1405, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Zhou, T.T.; Jin, H.; Jing, T.; Song, B.; Zhou, Y.K.; Mei, S.R.; Lee, Y.I. Rapid and selective extraction of multiple macrolide antibiotics in foodstuff samples based on magnetic molecularly imprinted polymers. Talanta 2015, 137. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yin, Y.; Gan, M.; Meng, M.; Dai, X.; Wu, R.; Shi, W.; Yan, Y. Fabrication and evaluation of molecularly imprinted multi-hollow microspheres adsorbents with tunable inner pore structures derived from templating Pickering double emulsions. Chem. Eng. J. 2015, 266, 299–308. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Y.; Li, J.J.; Su, X.M.; Wu, Z.Y.; Li, P.F.; Lei, F.H.; Tan, X.C.; Shi, Z.W. Synthesis of magnetic molecularly imprinted polymers for the selective separation and determination of metronidazole in cosmetic samples. Anal. Bioanal. Chem. 2015, 407, 3875–3880. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Aboufazeli, F.; Zhad, H.; Sadeghi, O.; Najafi, E. Determination of Sulfonamides in Chicken Meat by Magnetic Molecularly Imprinted Polymer Coupled to HPLC-UV. Food Anal. Methods 2014, 7, 73–80. [Google Scholar] [CrossRef]
- Fan, J.-P.; Xu, X.-K.; Xu, R.; Zhang, X.-H.; Zhu, J.-H. Preparation and characterization of molecular imprinted polymer functionalized with core/shell magnetic particles (Fe3O4@SiO2@MIP) for the simultaneous recognition and enrichment of four taxoids in Taxus × media. Chem. Eng. J. 2015, 279, 567–577. [Google Scholar] [CrossRef]
- Gao, R.X.; Kong, X.; Su, F.H.; He, X.W.; Chen, L.X.; Zhang, Y.K. Synthesis and evaluation of molecularly imprinted core-shell carbon nanotubes for the determination of triclosan in environmental water samples. J. Chromatogr. A 2010, 1217, 8095–8102. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, R.; He, X.; Chen, L.; Zhang, Y. Synthesis and characterization of the core-shell magnetic molecularly imprinted polymers (Fe3O4@MIPs) adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J. Chromatogr. A 2012, 1245, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.








| Analytes | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|
| n | KF | R2 | qm (mg∙g−1) | KL (L∙mg−1) | R2 | |
| S-(−)-OFL | 1.279 | 0.833 | 0.9926 | 106.383 | 0.004 | 0.9507 |
| R-(+)-OFL | 1.274 | 0.824 | 0.9921 | 107.527 | 0.004 | 0.9523 |
| Model | Analytes | Equations | k | qe | qe,c | R2 |
|---|---|---|---|---|---|---|
| Pseudo-first-order model | S-(−)-OFL | ln(qe − qt) = 0.551 − 0.144t | 0.144 | 0.715 | 0.579 | 0.8458 |
| R-(+)-OFL | ln(qe − qt) = 0.517 − 0.138t | 0.138 | 0.712 | 0.598 | 0.8688 | |
| Pseudo-second-order model | S-(−)-OFL | t/qt = 1.399t + 7.744 | 0.253 | 0.715 | 0.701 | 0.9929 |
| R-(+)-OFL | t/qt = 1.404t + 7.945 | 0.248 | 0.712 | 0.708 | 0.9901 |
| Analytes | qMIP (μg∙g−1) | qNIP (μg∙g−1) | KMIP (mL∙g−1) | KNIP (mL∙g−1) | α |
|---|---|---|---|---|---|
| S-(−)-OFL | 1455.83 | 821.90 | 69.72 | 24.50 | 2.85 |
| R-(+)-OFL | 1449.15 | 824.00 | 68.95 | 24.59 | 2.80 |
| Analytes | OFL Added (μg∙g−1) | Recovery (%) | Precision (RSD%) | |
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| S-(−)-OFL | 0.25 | 79.3 | 3.5 | 6.0 |
| 1.25 | 83.7 | 2.9 | 3.4 | |
| 2.5 | 84.1 | 3.9 | 4.2 | |
| R-(+)-OFL | 0.25 | 79.2 | 3.6 | 5.6 |
| 1.25 | 83.9 | 3.2 | 3.5 | |
| 2.5 | 84.4 | 4.0 | 4.6 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-F.; Jin, H.-X.; Wang, Y.-G.; Yang, L.-Y.; OuYang, X.-K.; Wu, W.-J. Synthesis and Characterization of Magnetic Molecularly Imprinted Polymer for the Enrichment of Ofloxacin Enantiomers in Fish Samples. Molecules 2016, 21, 915. https://doi.org/10.3390/molecules21070915
Wang Y-F, Jin H-X, Wang Y-G, Yang L-Y, OuYang X-K, Wu W-J. Synthesis and Characterization of Magnetic Molecularly Imprinted Polymer for the Enrichment of Ofloxacin Enantiomers in Fish Samples. Molecules. 2016; 21(7):915. https://doi.org/10.3390/molecules21070915
Chicago/Turabian StyleWang, Yan-Fei, Huo-Xi Jin, Yang-Guang Wang, Li-Ye Yang, Xiao-Kun OuYang, and Wei-Jian Wu. 2016. "Synthesis and Characterization of Magnetic Molecularly Imprinted Polymer for the Enrichment of Ofloxacin Enantiomers in Fish Samples" Molecules 21, no. 7: 915. https://doi.org/10.3390/molecules21070915





