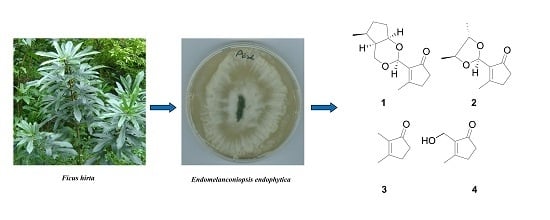
Two New Secondary Metabolites from the Endophytic Fungus Endomelanconiopsis endophytica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of New Compounds
2.2. Cytotoxicity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sati, S.C.; Savita, J. Endophytic fungal associations of plants and antioxidant compounds. In Plants as a Source of Natural Antioxidants; Dubey, N.K., Ed.; CAB International: Wallingford, UK, 2015; pp. 245–251. [Google Scholar]
- Aly, A.; Debbab, H.A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Micribiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes-secret producers of bioactive plant metabolites. Pharmazie 2013, 68, 499–505. [Google Scholar] [PubMed]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.M.; Eparvier, V.; Mandavid, H.; Bendelac, A.; Odonne, G.; Dayan, L.; Duplais, C.; Espindola, L.S.; Stien, D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry 2013, 96, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Zhang, Y.; Chen, X.Z.; Zhang, N.; Liu, Y.B. Metabolites Produced by the Endophytic Fungus Aspergillus fumigatus from the stem of Erythrophloeum fordii Oliv. Molecules 2015, 20, 10793–10799. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, F.; Che, Y.; Liu, G.; Liu, L. New Bergamotane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Molecules 2015, 20, 14611–14620. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Kusari, S.; Laatsch, H.; Golz, C.; Kusari, P.; Strohmann, C.; Kayser, O.; Spiteller, M. Antibacterial azaphilones from an endophytic fungus, Colletotrichum sp. BS4. J. Nat. Prod. 2016, 79, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.N.; Tang, L.H.; Huang, S.C.; Liu, D.D.; Wang, Y.; Liu, L.F.; Lai, P.; Ye, M.R. Study on the antitussive and antiasthmatic effects of Radix Fici Hirtae. J. Chin. Med. Mater. 2009, 32, 571–574. [Google Scholar]
- Tao, M.H.; Chen, Y.C.; Wei, X.Y.; Tan, J.W.; Zhang, W.M. Chemical constituents of the endophytic fungus Phomopsis sp. A240 isolated from Taxus chinensis var. mairei. Helv. Chim. Acta 2014, 97, 426–430. [Google Scholar] [CrossRef]
- Li, D.L.; Chen, Y.C.; Tao, M.H.; Li, H.H.; Zhang, W.M. Two new octahydronaphthalene derivatives from Trichoderma spirale, an endophytic fungus derived from Aquilaria sinensis. Helv. Chim. Acta 2012, 95, 805–809. [Google Scholar] [CrossRef]
- Sun, Z.H.; Liang, F.L.; Wu, W.; Chen, Y.C.; Pan, Q.L.; Li, H.H.; Ye, W.; Liu, H.X.; Li, S.N.; Tan, G.H.; et al. Guignardones P-S, New meroterpenoids from the endophytic fungus Guignardia mangiferae A348 derived from the medicinal plant Smilax glabra. Molecules 2015, 20, 22900–22907. [Google Scholar] [CrossRef] [PubMed]
- Rausch, B.J.; Gleiter, R.; Rominger, F. Synthesis and cyclic voltammetry of 1,2,3-trisubstituted bis(cyclopentadienyl)zirconium dichlorides. J. Organomet. Chem. 2002, 658, 242–250. [Google Scholar] [CrossRef]
- Vatele, J.M. Lewis acid-catalyzed oxidative rearrangement of tertiary allylic alcohols mediated by TEMPO. Tetrahedron 2010, 66, 904–912. [Google Scholar] [CrossRef]
- Chomcheon, P.; Sriubolmas, N.; Wiyakrutta, S.; Ngamrojanavanich, N.; Chaichit, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cyclopentenones, scaffolds for organic syntheses produced by the endophytic fungus mitosporic dothideomycete sp. LRUB20. J. Nat. Prod. 2006, 69, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screenimg. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Rojas, E.I.; Herre, E.A.; Mejía, L.C.; Arnold, A.E.; Chaverri, P.; Samuels, G.J. Endomelanconiopsis, a new anamorph genus in the Botryosphaeriaceae. Mycologia 2008, 100, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Liang, F.L.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Two new xyloketals from the endophytic fungus Endomelanconiopsis endophytica derived from medicinal plant Ficus hirta. J. Asian Nat. Prod. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.

 ), HMBC (
), HMBC (  ), and NOE (
), and NOE (  ) correlations of compounds 1–2.
) correlations of compounds 1–2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 206.8 | 207.1 | ||
| 2 | 137.4 | 136.0 | ||
| 3 | 177.8 | 177.5 | ||
| 4 | 2.52 (1H, m) 2.41 (1H, m) | 32.4 | 2.55 (2H, ddd, 5.6, 2.3, 1.1) | 32.5 |
| 5 | 2.36 (1H, m) | 34.4 | 2.37 (1H, m) | 34.5 |
| 6 | 5.36 (1H, s) | 94.4 | 5.80 (1H, s) | 95.9 |
| 7 | 2.31 (3H, s) | 18.3 | 2.25 (3H, s) | 17.8 |
| 1′ | 4.25 (1H, m) | 81.0 | 1.27 (3H, d, 6.1) | 17.3 |
| 2′ | 1.18 (1H, ddd, 10.7, 4.5, 3.0) | 47.2 | 3.82 (1H, dd, 7.8, 6.1) | 78.6 |
| 3′ | 2.42 (1H, m) | 32.5 | 3.70 (1H, dd, 7.8, 6.0) | 80.4 |
| 4′ | 2.12 (1H, m) 1.25 (1H, m) | 31.8 | 1.36 (3H, d, 6.0) | 16.5 |
| 5′ | 1.70 (1H, ddd, 14.3, 9.1, 4.9) 1.87 (1H, m) | 31.4 | ||
| 6′ | 4.04 (1H, d, 11.8) 4.07 (1H, dd, 11.8, 3.0) | 66.3 | ||
| 7′ | 1.04 (3H, d, 6.7) | 19.0 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-H.; Li, H.-H.; Liang, F.-L.; Chen, Y.-C.; Liu, H.-X.; Li, S.-N.; Tan, G.-H.; Zhang, W.-M. Two New Secondary Metabolites from the Endophytic Fungus Endomelanconiopsis endophytica. Molecules 2016, 21, 943. https://doi.org/10.3390/molecules21070943
Sun Z-H, Li H-H, Liang F-L, Chen Y-C, Liu H-X, Li S-N, Tan G-H, Zhang W-M. Two New Secondary Metabolites from the Endophytic Fungus Endomelanconiopsis endophytica. Molecules. 2016; 21(7):943. https://doi.org/10.3390/molecules21070943
Chicago/Turabian StyleSun, Zhang-Hua, Hao-Hua Li, Fa-Liang Liang, Yu-Chan Chen, Hong-Xin Liu, Sai-Ni Li, Guo-Hui Tan, and Wei-Min Zhang. 2016. "Two New Secondary Metabolites from the Endophytic Fungus Endomelanconiopsis endophytica" Molecules 21, no. 7: 943. https://doi.org/10.3390/molecules21070943
APA StyleSun, Z. -H., Li, H. -H., Liang, F. -L., Chen, Y. -C., Liu, H. -X., Li, S. -N., Tan, G. -H., & Zhang, W. -M. (2016). Two New Secondary Metabolites from the Endophytic Fungus Endomelanconiopsis endophytica. Molecules, 21(7), 943. https://doi.org/10.3390/molecules21070943