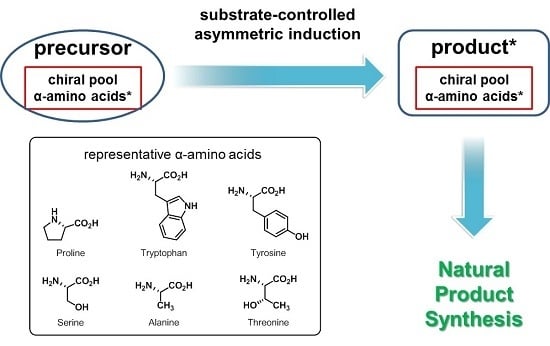
Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis
Abstract
:1. Introduction
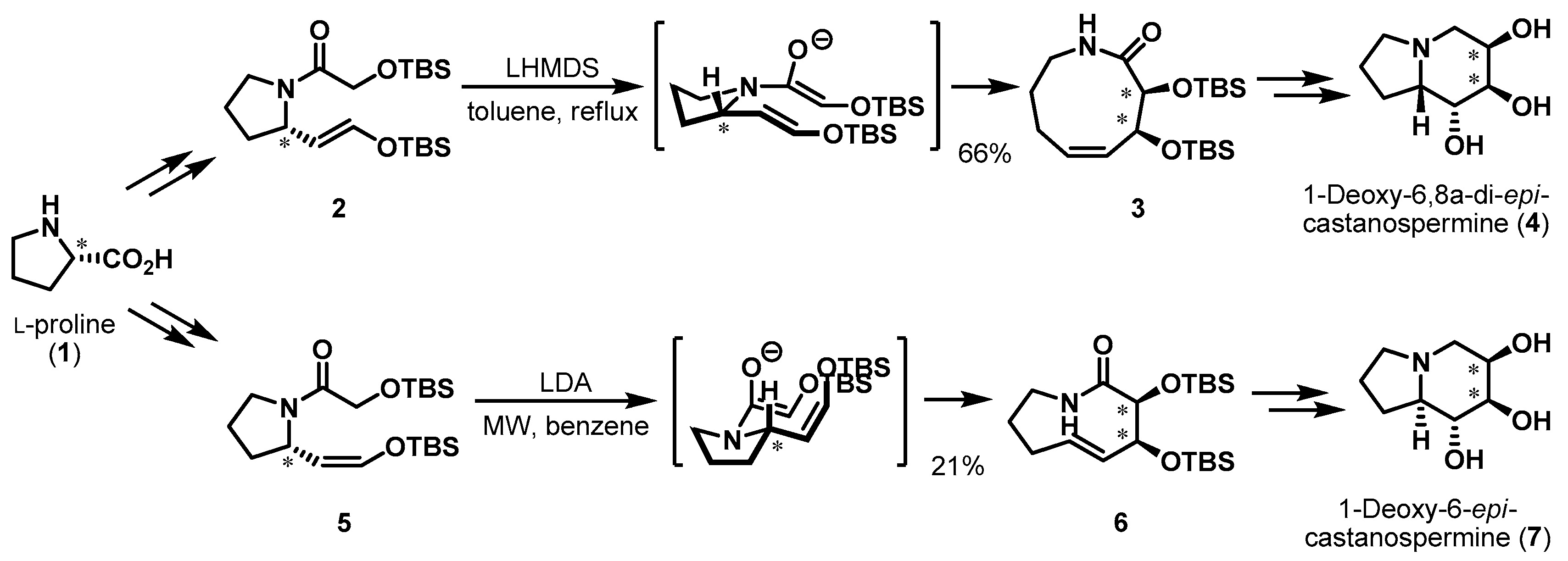
2. Chiral Pool: Proline
3. Chiral Pool: Tryptophan
4. Chiral Pool: Tyrosine
5. Chiral Pool: Serine
6. Chiral Pool: Alanine
7. Chiral Pool: Threonine
8. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Acetyl |
| Ala | Alanine |
| BBN | Borabicyclo[3.3.1]nonane |
| Bn | Benzyl |
| Boc | t-Butoxycarbonyl |
| Bu | Butyl |
| Cbz | Benzyloxycarbonyl |
| cod | 1,5-Cyclooctadiene |
| DCE | 1,1-Dichloroethane |
| DCC | N,N'-Dicyclohexylcarbodiimide |
| DIBAL-H | Diisobutylaluminum hydride |
| DMAP | N,N-4-Dimethylaminopyridine |
| DME | 1,2-Dimethoxyethane |
| DTBP | 2,6-Di-tert-butylpyridine |
| Et | Ethyl |
| Fmoc | 9-Fluorenylmethoxycarbonyl |
| HATU | O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate |
| KHMDS | Potassium bis(trimethylsilyl)amide |
| LDA | Lithium diisopropylamide |
| Leu | Leucine |
| LHMDS | Lithium bis(trimethylsilyl)amide |
| Me | Methyl |
| Mes | Mesityl |
| MS | Molecular sieves |
| MW | Microwave |
| NMO | N-Methylmorpholine N-oxide |
| Phe | Phenylalanine |
| TBAI | Tetra-n-butylammonium iodide |
| TBDPS | t-Butyldiphenylsilyl |
| TBS | t-Butyldimethylsilyl |
| Tf | Trifluoromethanesulfonyl |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TIPS | Triisopropylsilyl |
| Trp | Tryptophan |
| Ts | p-toluenesulfonyl |
References
- Casiraghi, G.; Zanardi, F. Stereoselective Approaches to Bioactive Carbohydrates and Alkaloids-With a Focus on Recent Syntheses Drawing from the Chiral Pool. Chem. Rev. 1995, 95, 1677–1716. [Google Scholar] [CrossRef]
- Rouf, A.; Taneja, S.C. Synthesis of Single-enantiomer Bioactive Molecules: A Brief Overview. Chirality 2014, 26, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.-U. The Chiral Pool as a Source of Enantioselective Catalysts and Auxiliaries. Chem. Rev. 1992, 92, 835–852. [Google Scholar] [CrossRef]
- Nugent, W.A.; RajanBabu, T.V.; Burk, M.J. Beyond Nature’s Chiral Pool: Enantioselective Catalysis in Industry. Science 1993, 259, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Goti, A.; Cicchi, S.; Cordero, F.M.; Fedi, V.; Brandi, A. A Straightforward Route to Enantiopure Pyrrolizidines and Indolizidines by Cycloaddition to Pyrroline N-Oxides Derived from the Chiral Pool. Molecules 1999, 4, 1–12. [Google Scholar] [CrossRef]
- Haleema, S.; Vavan Sasi, P.; Ibnusaud, I.; Polavarapu, P.L.; Kagan, H.B. Enantiomerically pure compounds related to chiral hydroxy acids derived from renewable resources. RSC Adv. 2012, 2, 9257–9285. [Google Scholar] [CrossRef]
- Singh, P.; Samanta, K.; Das, S.K.; Panda, G. Amino acid chirons: a tool for asymmetric synthesis of heterocycles. Org. Biomol. Chem. 2014, 12, 6297–6339. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, J.; Sim, J.; Lee, S.; Han, Y.T.; Chang, D.-J.; Kim, D.-D.; Suh, Y.-G. Asymmetric Syntheses of 1-Deoxy-6,8a-di-epi-castanospermine and 1-Deoxy-6-epi-castanospermine. J. Org. Chem. 2012, 77, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Winchester, B.G.; Cenci di Bello, I.; Richardson, A.C.; Nash, R.J.; Fellows, L.E.; Ramsden, N.G.; Fleet, G. The structural basis of the inhibition of human glycosidases by castanospermine analogues. Biochem. J. 1990, 269, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.-M.; Kim, N.-J.; Shin, D.; Jung, J.-K.; Jung, J.-W.; Chang, D.-J.; Moon, H.; Suh, Y.-G. A Concise Total Synthesis of (+)-Tetrabenazine and (+)-α-Dihydrotetrabenazine. Chem. Eur. J. 2010, 16, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Inanaga, J.; Yamaguchi, M. Use of 1,8-Diazabicyclo[5.4.0]undec-7-ene in Preparation of Trimethylsilyl Enol Ethers and Trimethylsilylacetylenes. Bull. Chem. Soc. Jpn. 1981, 54, 3229–3230. [Google Scholar] [CrossRef]
- Reddy, A.S.; Srihari, P. A facile approach to the synthesis of securinega alkaloids: stereoselective total synthesis of (−)-allonorsecurinine. Tetrahedron Lett. 2012, 53, 5926–5928. [Google Scholar] [CrossRef]
- Mercado-Marin, E.V.; Garcia-Reynaga, P.; Romminger, S.; Pimenta, E.F.; Romney, D.K.; Lodewyk, M.W.; Williams, D.E.; Andersen, R.J.; Miller, S.J.; Tantillo, D.J.; et al. Total synthesis and isolation of citrinalin and cyclopiamine congeners. Nature 2014, 509, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.F.; Boeyens, J.C.A.; Holzapfel, C.W.; Steyn, P.S. Cyclopiamines A and B, novel oxindole metabolites of Penicillium cyclopium westling. J. Chem. Soc. Perkin Trans. 1 1979, 1751–1761. [Google Scholar] [CrossRef]
- Pimenta, E.F.; Vita-Marques, A.M.; Tininis, A.; Seleghim, M.H.R.; Sette, L.D.; Veloso, K.; Ferreira, A.G.; Williams, D.E.; Patrick, B.O.; Dalisay, D.S.; Andersen, R.J.; Berlinck, R.G.S. Use of Experimental Design for the Optimization of the Production of New Secondary Metabolites by Two Penicillium Species. J. Nat. Prod. 2010, 73, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Nakatsubo, F.; Aratani, M.; Goto, T.; Inoue, S.; Kakoi, H. Synthetic approach towards tetrodotoxin. I. Diels-Alder reaction of α-oximinoethylbenzoquinones with butadiene. Tetrahedron Lett. 1970, 11, 5127–5128. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Kim, S. Biomimetic Total Synthesis of (−)-Penibruguieramine A Using Memory of Chirality and Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2015, 54, 10875–10878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-F.; Kurtán, T.; Yang, X.-H.; Mándi, A.; Geng, M.-Y.; Ye, B.-P.; Taglialatela-Scafati, O.; Guo, Y.-W. Penibruguieramine A, a Novel Pyrrolizidine Alkaloid from the Endophytic Fungus Penicillium sp. GD6 Associated with Chinese Mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, M.E.; Chuang, K.V.; Reisman, S.E. Copper-Catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)-Naseseazines A and B. J. Am. Chem. Soc. 2013, 135, 5557–5560. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Conte, M.; Aalbersberg, W.G.L.; Feussner, K.; Capon, R.J. Naseseazines A and B: A New Dimeric Diketopiperazine Framework from a Marine-Derived Actinomycete, Streptomyces sp. Org. Lett. 2009, 11, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Yum, E.K. Synthesis of Indoles via Palladium-Catalyzed Heteroannulation of Internal Alkynes. J. Am. Chem. Soc. 1991, 113, 6690–6692. [Google Scholar] [CrossRef]
- Garfunkle, J.; Kimball, F.S.; Trzupek, J.D.; Takizawa, S.; Shimamura, H.; Tomishima, M.; Boger, D.L. Total Synthesis of Chloropeptin II (Complestatin) and Chloropeptin I. J. Am. Chem. Soc. 2009, 131, 16036–16038. [Google Scholar] [CrossRef] [PubMed]
- Ruchti, J.; Carreira, E.M. Ir-Catalyzed Reverse Prenylation of 3-Substituted Indoles: Total Synthesis of (+)-Aszonalenin and (−)-Brevicompanine B. J. Am. Chem. Soc. 2014, 136, 16756–16759. [Google Scholar] [CrossRef] [PubMed]
- Diebolt, O.; Tricas, H.; Freixa, Z.; van Leeuwen, P.W.N.M. Strong π-Acceptor Ligands in Rhodium-Catalyzed Hydroformylation of Ethene and 1-Octene: Operando Catalysis. ACS Catal. 2013, 3, 128–137. [Google Scholar] [CrossRef]
- Kusano, M.; Sotoma, G.; Koshino, H.; Uzawa, J.; Chijimatsu, M.; Fujioka, S.; Kawano, T.; Kimura, Y. Brevicompanines A and B: New plant growth regulators produced by the fungus, Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1 1998, 1, 2823–2826. [Google Scholar] [CrossRef]
- Kimura, Y.; Hamasaki, T.; Nakajima, H.; Isogai, A. Structure of aszonalenin, a new metabolite of aspergillus zonatus. Tetrahedron Lett. 1982, 23, 225–228. [Google Scholar] [CrossRef]
- Feng, Y.; Holte, D.; Zoller, J.; Umemiya, S.; Simke, L.R.; Phil, S.B. Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C-H Borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kirksey, J.W.; Moore, J.H.; Blankenship, B.R.; Diener, U.L.; Davis, N.D. Tremorgenic Toxin from Penicillium verruculosum. Appl. Microbiol. 1972, 24, 248–250. [Google Scholar] [PubMed]
- Yamazaki, M.; Suzuki, S.; Miyaki, K. Tremorgenic Toxins from Aspergillus fumigatus FRES. Chem. Pharm. Bull. 1971, 19, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Shade, R.E.; Hyde, A.M.; Olsen, J.-C.; Merlic, C.A. Copper-Promoted Coupling of Vinyl Boronates and Alcohols: A Mild Synthesis of Allyl Vinyl Ethers. J. Am. Chem. Soc. 2010, 132, 1202–1203. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Kurogi, T.; Okaya, S.; Okano, K.; Tokuyama, H. Total Synthesis of (−)-Acetylaranotin. Angew. Chem. Int. Ed. 2012, 51, 13062–13065. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Huckstep, L.L.; Lively, D.H.; DeLong, D.C.; Marsh, M.M.; Neuss, N. Aranotin and Related Metabolites from Avachniofus Aureus I. Determination of Structure. J. Am. Chem. Soc. 1968, 90, 2980–2982. [Google Scholar] [CrossRef]
- Wipf, P.; Kim, Y.; Goldstein, D.M. Asymmetric Total Synthesis of the Stemona Alkaloid (−)-Stenine. J. Am. Chem. Soc. 1995, 117, 11106–11112. [Google Scholar] [CrossRef]
- Krishna, P.R.; Reddy, B.K. Total Synthesis of a Pyrrolidin-2-one with the Structure Proposed for the Alkaloid Rigidiusculamide A. Helv. Chim. Acta 2013, 96, 1564–1570. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Niu, S.; Zhuang, W.; Che, Y. Pyrrolidinones from the Ascomycete Fungus Albonectria rigidiuscula. J. Nat. Prod. 2009, 72, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Vanrheenen, V.; Kelly, R.C.; Cha, D.Y. An improved catalytic OsO4 oxidation of olefin to cis-1,2-glycols using tertiary amine oxides as the oxidant. Tetrahedron Lett. 1976, 17, 1973–1976. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, B.; Li, Y. Total Synthesis of (−)-(α)-Kainic Acid via a Diastereoselective Intramolecular [3 + 2] Cycloaddition Reaction of an Aryl Cyclopropyl Ketone with an Alkyne. Org. Lett. 2012, 14, 2540–2543. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Takemoto, T.; Shimizu, Z. The effective principle of Digenea simplex Aq. I. Separation of the effective fraction by liquid chromatography. J. Pharm. Soc. Jpn. 1953, 73, 1026–1028. [Google Scholar]
- Matveenko, M.; Liang, G.; Lauterwasser, E.M.W.; Zubía, E.; Trauner, D. A Total Synthesis Prompts the Structure Revision of Haouamine B. J. Am. Chem. Soc. 2012, 134, 9291–9295. [Google Scholar] [CrossRef] [PubMed]
- Garrido, L.; Zubía, E.; Ortega, M.J.; Salvá, J. Haouamines A and B: A New Class of Alkaloids from the Ascidian Aplidium haouarianum. J. Org. Chem. 2003, 68, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Paladino, M.; Zaifman, J.; Ciufolini, M.A. Total Synthesis of (+)-3-Demethoxyerythratidinone and (+)-Erysotramidine via the Oxidative Amidation of a Phenol. Org. Lett. 2015, 17, 3422–3425. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Furukawa, H.; Haruna, M. Studies on the Erythrina Alkaloids. VII. Alkaloids of Erythrina arborescens ROXB. (2). Structures of New Alkaloids, Erysotramidine, Erytharbine and 11-Hydroxyerysotrine. Yakugaku Zasshi 1973, 93, 1617–1621. [Google Scholar] [PubMed]
- Wipf, P.; Miller, C.P. A New Synthesis of Highly Functionalized Oxazoles. J. Org. Chem. 1993, 58, 3604–3606. [Google Scholar] [CrossRef]
- Chow, Y.L.; Colòn, C.J.; Tan, J.N.S. A(1,3) interaction and conformational energy of axial-axial 1,3-dimethyl interaction. Can. J. Chem. 1968, 46, 2821–2825. [Google Scholar] [CrossRef]
- Lunazzi, L.; Macciantelli, D.; Tassi, D.; Dondoni, A. Conformational studies by dynamic nuclear magnetic resonance. Part 17. Stereodynamic processes in hindered piperidyl-amides and -amidines. J. Chem. Soc. Perkin Trans. 2 1980, 717–723. [Google Scholar] [CrossRef]
- Gouault, N.; Le Roch, M.; de Campos Pinto, G.; David, M. Total synthesis of dendrobate alkaloid (+)-241D, isosolenopsin and isosolenopsin A: application of a gold-catalyzed cyclization. Org. Biomol. Chem. 2012, 10, 5541–5546. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.W.; Daly, J.W.; Myers, C.W. Alkaloids from a Panamanian Poison Frog, Dendrobates speciosus: Identification of Pumiliotoxin-A and Allo-pumiliotoxin Class Alkaloids, 3,5-Disubstituted Indolizidines, 5-Substituted 8-Methylindolizidines, and a 2-Methyl-6-nonyl-4-hydroxypiperidine. J. Nat. Prod. 1988, 51, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Daloze, D.; Braekman, J.C. Synthesis of the Fire Ant Alkaloids, Solenopsins. A Review. Org. Prep. Proced. Int. 1996, 28, 499–543. [Google Scholar] [CrossRef]
- Gouault, N.; le Roch, M.; Cheignon, A.; Uriac, P.; David, M. Enantiospecific Synthesis of Pyridinones as Versatile Intermediates toward Asymmetric Piperidines. Org. Lett. 2011, 13, 4371–4373. [Google Scholar] [CrossRef] [PubMed]
- Matthies, S.; Stallforth, P.; Seeberger, P.H. Total Synthesis of Legionaminic Acid as Basis for Serological Studies. J. Am. Chem. Soc. 2015, 137, 2848–2851. [Google Scholar] [CrossRef] [PubMed]
- Zunk, M.; Kiefel, M.J. The occurrence and biological significance of the α-keto-sugars pseudaminic acid and legionaminic acid within pathogenic bacteria. RSC Adv. 2014, 4, 3413–3421. [Google Scholar] [CrossRef]
- Liang, X.; Lee, C.-J.; Chen, X.; Chung, H.S.; Zeng, D.; Raetz, C.R.H.; Li, Y.; Zhou, P.; Toone, E.J. Syntheses, structures and antibiotic activities of LpxC inhibitors based on the diacetylene scaffold. Bioorg. Med. Chem. 2011, 19, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Szechner, B.; Achmatowicz, O.; Galdecki, Z.; Fruziński, A. Synthesis and absolute configuration of four diastereoisomeric 1-(2-furyl)-2-aminobutane-1,3-diols. Tetrahedron 1994, 50, 7611–7624. [Google Scholar] [CrossRef]

















© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paek, S.-M.; Jeong, M.; Jo, J.; Heo, Y.M.; Han, Y.T.; Yun, H. Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis. Molecules 2016, 21, 951. https://doi.org/10.3390/molecules21070951
Paek S-M, Jeong M, Jo J, Heo YM, Han YT, Yun H. Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis. Molecules. 2016; 21(7):951. https://doi.org/10.3390/molecules21070951
Chicago/Turabian StylePaek, Seung-Mann, Myeonggyo Jeong, Jeyun Jo, Yu Mi Heo, Young Taek Han, and Hwayoung Yun. 2016. "Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis" Molecules 21, no. 7: 951. https://doi.org/10.3390/molecules21070951
APA StylePaek, S. -M., Jeong, M., Jo, J., Heo, Y. M., Han, Y. T., & Yun, H. (2016). Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis. Molecules, 21(7), 951. https://doi.org/10.3390/molecules21070951