Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Results
2.1. TFs Protects NRK-52E Cells from H/R Injury
2.2. TFs Reduces ROS Levels in NRK-52E Cells
2.3. TFs Improves Renal I/R Injury in Rats
2.4. TFs Attenuates Oxidative Stress in Vivo
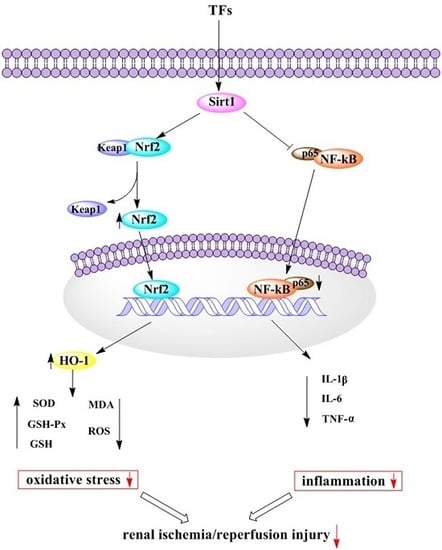
2.5. TFs Activates Sirt1/Nrf2 Signaling Pathway in Vitro and Vivo
2.6. TFs Attenuates Inflammation in Vitro and Vivo
2.7. Sirt1 siRNA Abrogates the Nephroprotective Effects of the TFs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Herbal Material and the Total Flavonoids
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Measurement of Intracellular ROS
4.6. Animals
4.7. Measurement of BUN, Cr, MDA, SOD, GSH and GSH-Px Levels
4.8. Histopathologic and Immunofluorescence Assays
4.9. Real-Time PCR Assay
4.10. Western Blotting Assay
4.11. Sirt1 siRNA Transfection
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zuk, A.; Bonventre, J.V. Acute kidney injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xing, G.; Wang, L.; Wu, Y.; Li, S.; Xu, G.; He, Q.; Chen, J.; Chen, M.; Liu, X.; et al. Acute kidney injury in china: A cross-sectional survey. Lancet 2015, 386, 1465–1471. [Google Scholar] [CrossRef]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International society of nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; From pathophysiology to treatment. J. Renal Inj. Prev. 2015, 4, 20–27. [Google Scholar] [PubMed]
- Li, Y.; Zhong, D.; Lei, L.; Jia, Y.; Zhou, H.; Yang, B. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol. Biochem. 2015, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zheng, L.; Qi, Y.; Han, X.; Xu, Y.; Xu, L.; Yin, L.; Wang, C.; Zhao, Y.; Sun, H.; et al. Dioscin attenuates renal ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling pathway via up-regulation of HSP70. Pharmacol. Res. 2015, 100, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Honma, S. Regeneration of injured renal tubules. J. Pharmacol. Sci. 2014, 124, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Senbel, A.M.; AbdelMoneim, L.; Omar, A.G. Celecoxib modulates nitric oxide and reactive oxygen species in kidney ischemia/reperfusion injury and rat aorta model of hypoxia/reoxygenation. Vasc. Pharmacol. 2014, 62, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.N.; Kacan, M.; Unsal, D.; Sahan Firat, S.; Kemal Buharalioglu, C.; Vezir, O.; Korkmaz, B.; Cuez, T.; Canacankatan, N.; Sucu, N.; et al. Contribution of RhoA/Rho-kinase/MEK1/ERK1/2/iNOS pathway to ischemia/reperfusion-induced oxidative/nitrosative stress and inflammation leading to distant and target organ injury in rats. Eur. J. Pharmacol. 2014, 723, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Wang, L.T.; Huang, K.H.; Wang, C.C.; Chiang, C.K.; Liu, S.H. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J. Nutr. Biochem. 2014, 25, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Morales, D.; Escribese, M.M.; Stamatakis, K.; Garcia-Martos, M.; Alegre, L.; Conde, E.; Perez-Sala, D.; Mampaso, F.; Garcia-Bermejo, M.L. Requirements for proximal tubule epithelial cell detachment in response to ischemia: Role of oxidative stress. Exp. Cell Res. 2006, 312, 3711–3727. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Tan, J.; Li, M.; Song, S.; Miao, Y.; Zhang, Q. Sirt1: Role under the condition of ischemia/hypoxia. Cell Mol. Neurobiol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Marko, L.; Vigolo, E.; Hinze, C.; Park, J.K.; Roel, G.; Balogh, A.; Choi, M.; Wubken, A.; Cording, J.; Blasig, I.E.; et al. Tubular epithelial NF-kappaB activity regulates ischemic AKI. J. Am. Soc. Nephrol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jihong, Y.; Feng, Z.; Xiaomei, X.; Xiaohan, Z.; Guangzhi, W.; Zhenhai, M.; Dongyan, G.; Xiaochi, M.; Qing, F.; et al. Sirtuin 1-mediated inhibition of p66shc expression alleviates liver ischemia/reperfusion injury. Crit. Care Med. 2014, 42, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ynag, H.C.; You, L.; Wang, Y.Y.; He, W.J.; Hao, C.M. The histone deacetylase, sirt1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Tao, X.; Xu, Y.; Han, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Dioscin alleviates BDL- and DMN-induced hepatic fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway. Toxicol. Appl. Pharmacol. 2016, 292, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Wang, L.K.; Su, L.L.; Luo, C. COX-2 and Nrf2/ARE signaling pathways in anti-inflammation and antioxidation in vivo and in vitro. Chin. Bull. Life Sci. 2011, 23, 1027–1033. [Google Scholar]
- Luo, C.; Urgard, E.; Vooder, T.; Metspalu, A. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: Aging and anti-aging. Med. Hypotheses 2011, 77, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Huang, J.W.; Ding, P.Y.; Zang, H.G.; Kou, Z.J.; Li, T.; Fan, J.; Peng, Z.W.; Yan, W.J. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav. Brain Res. 2016, 309, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.R.; Donepudi, A.C.; Xu, J.; Wei, W.; Cheng, Q.C.; Driscoll, M.V.; Johnson, D.A.; Johnson, J.A.; Li, X.; Slitt, A.L. Fasting induces nuclear factor E2-related factor 2 and ATP-binding cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxid. Redox. Signal. 2014, 20, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Singh, S.; Aggarwal, B.B. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004, 279, 15096–15104. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Ungvari, Z.; Minor, R.K.; le Couteur, D.G.; de Cabo, R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 2012, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Jimenez, M.; Hurtado, O.; Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Pradillo, J.M.; McBurney, M.W.; Lizasoain, I.; Moro, M.A. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013, 44, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.C.; Lin, H.W.; Thompson, J.W.; Perez-Pinzon, M.A. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J. Cereb. Blood Flow Metab. 2011, 31, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, Y.R.; Zhong, X.; Deng, H.D.; Pu, L.; Peng, K.; Wang, L. Erythropoietin pretreatment ameliorates renal ischaemia-reperfusion injury by activating PI3K/Akt signalling. Nephrology 2015, 20, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Vanmassenhove, J.V.N.; van Biesen, W. Prevention and conservative management of acute kidney injury. Minerva Urol. Nefrol. 2016, 68, 58–71. [Google Scholar] [PubMed]
- Lv, J.; Feng, M.; Zhang, L.; Wan, X.; Zeng, Y.C.; Liang, P.F.; Xu, A.P. Protective effect of epigallo-catechin gallate, a major constituent of green tea, against renal ischemia-reperfusion injury in rats. Int. Urol. Nephrol. 2015, 47, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lu, M.; Ma, L.; Zhang, S.; Qiu, M.; Wang, Y. Osthole ameliorates renal ischemia-reperfusion injury in rats. J. Surg. Res. 2013, 183, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Huang, L.; Guo, E.; Li, R.; Yang, J.; Li, A.; Yang, Y.; Liu, S.; Hu, J.; Jiang, X.; et al. Baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, S.; Yin, L.; Tang, X.; Xu, Y.; Han, X.; Qi, Y.; Peng, J. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 62, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, L.; Xu, L.; Sun, H.; Li, H.; Yao, J.; Liu, K.; Peng, J. Subchronic toxicity study of the total flavonoids from Rosa laevigata michx fruit in rats. Regul. Toxicol. Pharmacol. 2012, 62, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, B.; Peng, J. Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem. 2011, 125, 719–725. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, B.; Han, X.; Xu, L.; Qi, Y.; Yin, L.; Xu, Y.; Zhao, Y.; Liu, K.; Peng, J. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 55, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.; Peng, J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, B.; Xu, L.; Yin, L.; Wang, X.; Peng, J.; Liu, K. The antioxidant activity and hypolipidemic activity of the total flavonoids from the fruit of Rosa laevigata Michx. Nat. Sci. 2010, 2, 175–183. [Google Scholar] [CrossRef]
- Yang, Y.; Song, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; Dong, Z. Renoprotective approaches and strategies in acute kidney injury. Pharmacol. Ther. 2016, 163, 58–73. [Google Scholar]
- Small, D.M.; Bennett, N.C.; Roy, S.; Gabrielli, B.G.; Johnson, D.W.; Gobe, G.C. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp. Nephrol. 2012, 122, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Patschan, D.; Patschan, S.; Muller, G.A. Inflammation and microvasculopathy in renal ischemia reperfusion injury. J. Transplant. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ji, L.; Zhang, S.; Xu, L.; Yin, L.; Li, L.; Zhao, Y.; Peng, J. Total flavonoids from Rosa laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hasegawa, K.; Itoh, H. Sirtuin and metabolic kidney disease. Kidney Int. 2015, 88, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.W.; Zhao, G.J.; Li, X.L.; Hong, G.L.; Li, M.F.; Qiu, Q.M.; Wu, B.; Lu, Z.Q. Sirt1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating Nrf2 in vitro. Int. J. Mol. Med. 2016, 37, 1049–1058. [Google Scholar] [PubMed]
- Li, S.; Zhao, G.; Chen, L.; Ding, Y.; Lian, J.; Hong, G.; Lu, Z. Resveratrol protects mice from paraquat-induced lung injury: The important role of Sirt1 and Nrf2 antioxidant pathways. Mol. Med. Rep. 2016, 13, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the sirt1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar]
- Sample Availability: Not available.





| Gene | GenBank | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| GAPDH | NM_017008.3 | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| IL-1β | NM_031512.2 | GACTTCACCATGGAACCCGT | CGAGACTGCCCATTCTCGAC |
| IL-6 | NM_012589.1 | ATTGTATGAACAGCGATGATGCAC | CCAGGTAGAAACGGAACTCCAGA |
| TNF-α | NM_012675.3 | TCAGTTCCATGGCCCAGAC | GTTGTCTTTGAGATCCATGCCATT |
| Primary Antibody * | Source | Dilution |
|---|---|---|
| Sirt1 | Rabbit | 1:1000 |
| Nrf2 | Rabbit | 1:1000 |
| Keap1 | Rabbit | 1:1000 |
| HO-1 | Rabbit | 1:1000 |
| p65 | Rabbit | 1:1000 |
| GAPDH | Rabbit | 1:1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Qi, Y.; Peng, J. Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules 2016, 21, 952. https://doi.org/10.3390/molecules21070952
Zhao L, Xu L, Tao X, Han X, Yin L, Qi Y, Peng J. Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules. 2016; 21(7):952. https://doi.org/10.3390/molecules21070952
Chicago/Turabian StyleZhao, Lisha, Lina Xu, Xufeng Tao, Xu Han, Lianhong Yin, Yan Qi, and Jinyong Peng. 2016. "Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation" Molecules 21, no. 7: 952. https://doi.org/10.3390/molecules21070952
APA StyleZhao, L., Xu, L., Tao, X., Han, X., Yin, L., Qi, Y., & Peng, J. (2016). Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules, 21(7), 952. https://doi.org/10.3390/molecules21070952