Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-cyclodextrin Conjugates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis of Mono[6-Deoxy-6-(Octadecanamido)]-β-CD (Stearamido-β-CD, S-β-CD) and Mono[6-Deoxy-6-(Octadecenamido)]-β-CD (Oleamido-β-CD, O-β-CD)
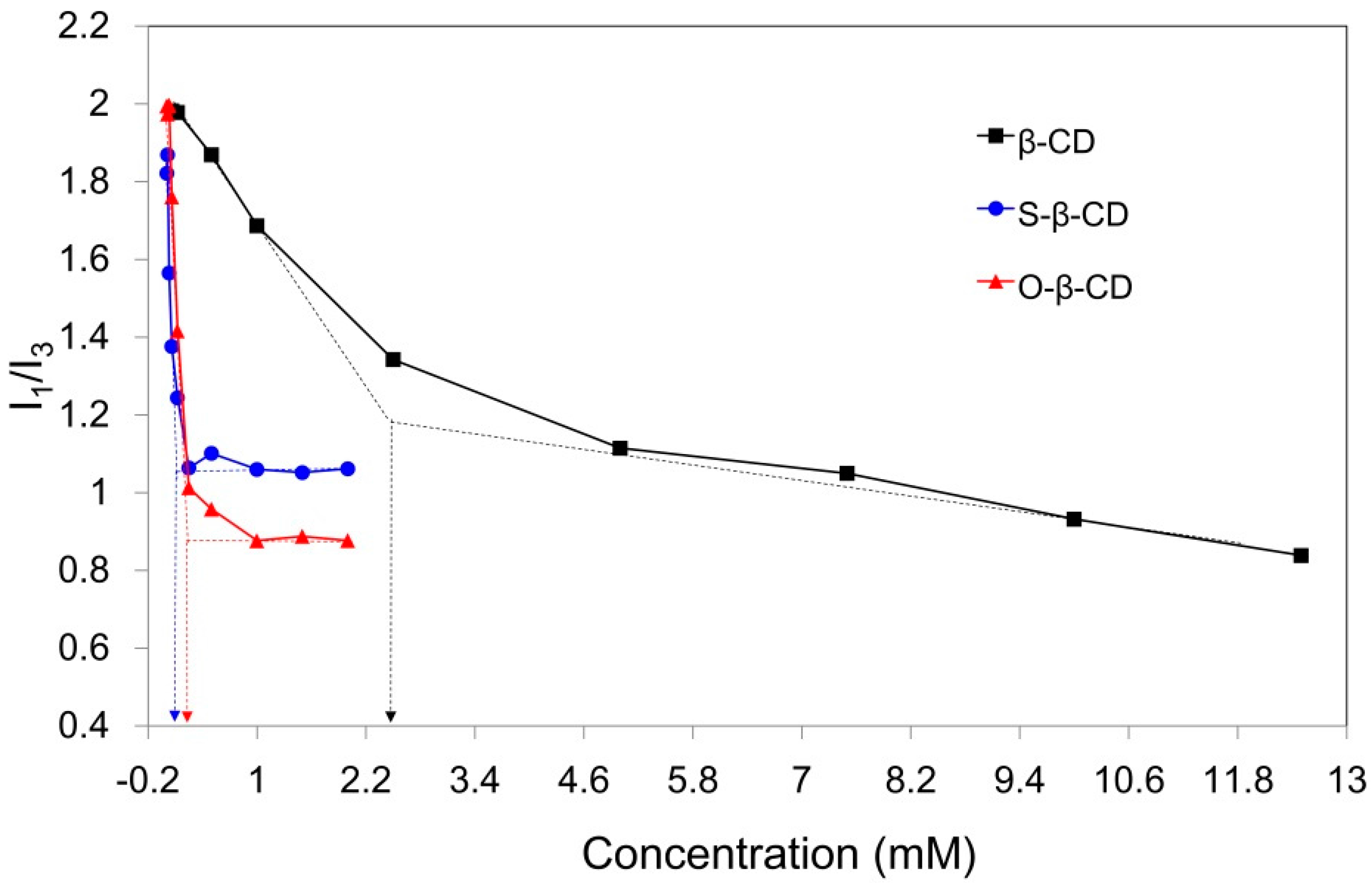
2.2. Self-Assembly of S-β-CD and O-β-CD in Water
2.3. Supramolecular Nano-Vesicles
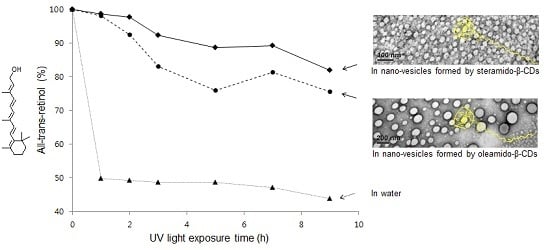
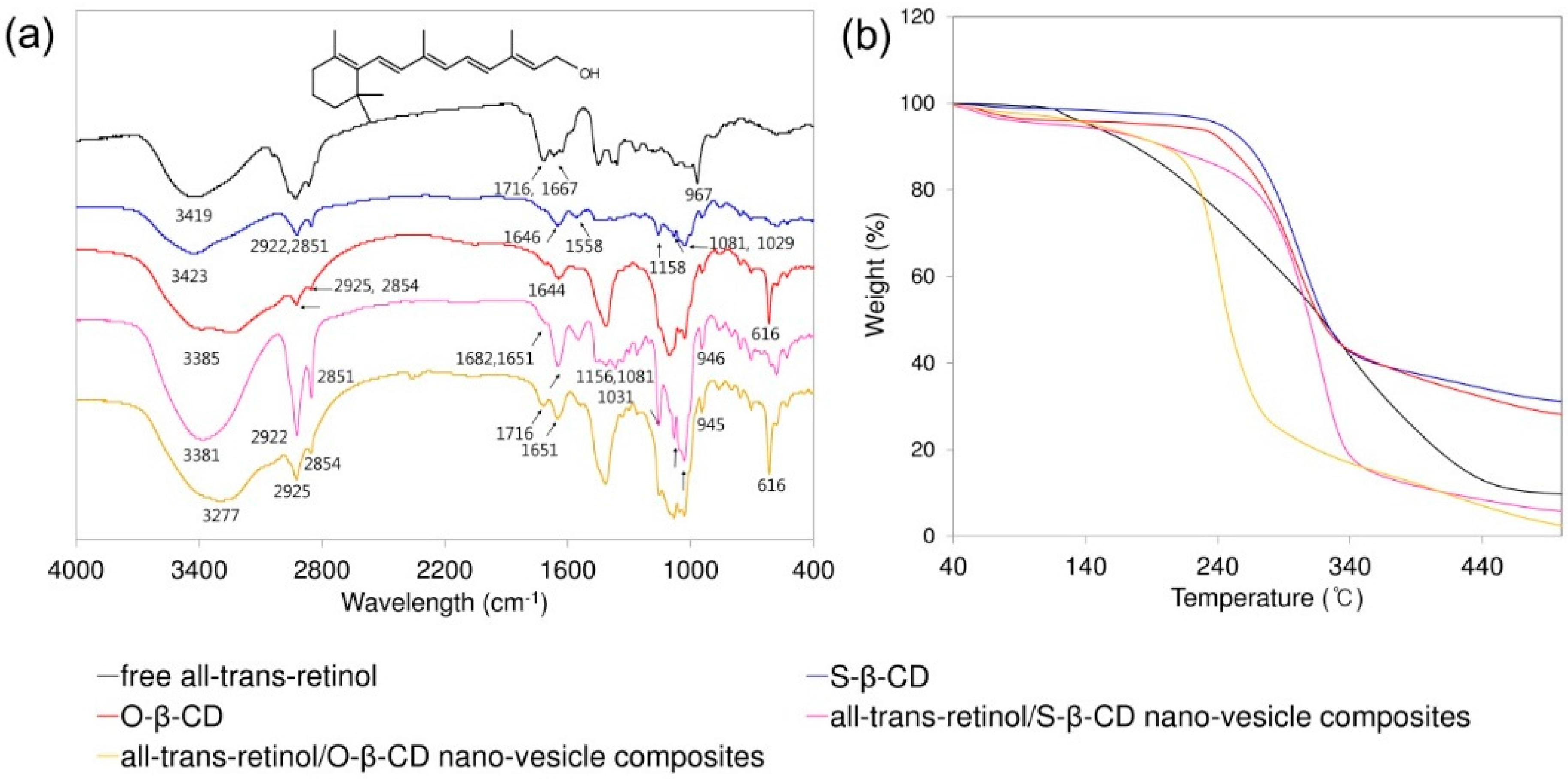
2.4. All-Trans-Retinol Encapsulation
2.5. All-Trans-Retinol Stabilization
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.2.1. N-Hydroxysuccinimide (NHS) Esters of Stearic Acid and Oleic Acid
3.2.2. Synthesis of S-β-CD and O-β-CD
3.3. Characterization
3.3.1. MALDI-TOF Mass Spectrometry
3.3.2. NMR Spectroscopy
3.4. Measurement of Fluorescence Spectroscopy
3.5. Aqueous Solubility of Fatty Amide-β-CD Conjugates
3.6. Characterization of Nano-Vesicles
3.6.1. DLS
3.6.2. TEM
3.7. Preparation of All-Trans-Retinol Encapsulated Nano-Vesicles
3.8. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.9. Thermogravimetric Analysis (TGA)
3.10. Photostability Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Cyclodextrin Technology; Springer Science & Business Media: Dordrecht, The Netherlands, 2013; Volume 1, pp. 79–185. [Google Scholar]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry; Springer Science & Business Media: Dordrecht, The Netherlands, 2012; Volume 6, pp. 68–78. [Google Scholar]
- Bai, Y.; Xu, G.-Y.; Sun, H.-Y.; Yang, X.-D.; Hao, A.-Y.; Pang, J.-Y.; Gong, H.-J.; Ao, M.-Q. The surface property and aggregation behavior of a hydrophobically modified cyclodextrin. Colloid Polym. Sci. 2010, 288, 415–421. [Google Scholar] [CrossRef]
- Sallas, F.; Darcy, R. Amphiphilic cyclodextrins-advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 2008, 957–969. [Google Scholar] [CrossRef]
- Zerkoune, L.; Angelova, A.; Lesieur, S. Nano-assemblies of modified cyclodextrins and their complexes with guest molecules: Incorporation in nanostructured membranes and amphiphile nanoarchitectonics design. Nanomaterials 2014, 4, 741–765. [Google Scholar] [CrossRef]
- Perret, F.; Parrot-Lopez, H. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons, Inc.: Hoboken, New Jersey, NJ, USA, 2011; pp. 197–233. [Google Scholar]
- Lee, E.; Jeong, K.-W.; Lee, J.; Shin, A.; Kim, J.-K.; Lee, J.; Lee, D. G.; Kim, Y. Structure-activity relationships of cecropin-like peptides and their interactions with phospholipid membrane. BMB Rep. 2013, 46, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.; Perly, B.; Djedaïni-Pilard, F. Self-assemblies of amphiphilic cyclodextrins. Eur. Biophys. J. 2007, 36, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Yun, D.; Jeong, D.; Im, J.; Kim, H.; Dindulkar, S.D.; Choi, Y.; Jung, S. Regioselective self-acylating cyclodextrins in organic solvent. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, N.; Perly, B. NMR investigations of the conformation of new cyclodextrin-based amphiphilic transporters for hydrophobic drugs: Molecular lollipops. J. Mol. Struct. 1992, 273, 215–226. [Google Scholar] [CrossRef]
- Noy, N. Physical-Chemical Properties and Action of Retinoids; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 1999; pp. 3–29. [Google Scholar]
- Bondi, A.; Sklan, D. Vitamin A and carotene in animal nutrition. Prog. Food Nutr. Sci. 1983, 8, 165–191. [Google Scholar]
- Hong, S.-H.; Kim, K.-R.; Oh, D.-K. Biochemical properties of retinoid-converting enzymes and biotechnological production of retinoids. Appl. Microbiol. Biotechnol. 2015, 99, 7813–7826. [Google Scholar] [CrossRef] [PubMed]
- Failloux, N.; Bonnet, I.; Perrier, E.; Baron, M.H. Effects of light, oxygen and concentration on vitamin A1. J. Raman Spectrosc. 2004, 35, 140–147. [Google Scholar] [CrossRef]
- Fu, P.; Cheng, S.-H.; Coop, L.; Xia, Q.; Culp, S.; Tolleson, W.; Wamer, W.; Howard, P. Photoreaction, phototoxicity, and photocarcinogenicity of retinoids. J. Environ. Sci. Health Pt. C Environ. Carcinog. Ecotoxicol. Rev. 2003, 21, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.-X.; Ren, T.; Fang, Y.-P.; Liu, Y.-C. Binding of vitamin A by β-cyclodextrin and heptakis (2, 6-O-dimethyl)-β-cyclodextrin. J. Inclusion Phenom. Mol. Recognit. Chem. 1995, 22, 251–256. [Google Scholar] [CrossRef]
- Lee, S.-C.; Yuk, H.-G.; Lee, D.-H.; Lee, K.-E.; Hwang, Y.-I.; Ludescher, R.D. Stabilization of retinol through incorporation into liposomes. J. Biochem. Mol. Biol. 2002, 35, 358–363. [Google Scholar] [PubMed]
- Lee, J.S.; Nam, Y.S.; Kang, B.Y.; Han, S.H.; Chang, I.S. Vitamin A microencapsulation within poly (methyl methacrylate)-g-polyethylenimine microspheres: Localized proton buffering effect on vitamin A stability. J. Appl. Polym. Sci. 2004, 92, 517–522. [Google Scholar] [CrossRef]
- Eskandar, N.G.; Simovic, S.; Prestidge, C.A. Chemical stability and phase distribution of all-trans-retinol in nanoparticle-coated emulsions. Int. J. Pharm. 2009, 376, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.; Rossatto, V.; Gallarate, M. Vitamin A and vitamin A palmitate stability over time and under UVA and UVB radiation. Int. J. Pharm. 2002, 240, 85–94. [Google Scholar] [CrossRef]
- Amiji, M.M. Pyrene fluorescence study of chitosan self-association in aqueous solution. Carbohydr. Polym. 1995, 26, 211–213. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py scale of solvent polarities. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
- Jun, B.-H.; Kim, G.; Baek, J.; Kang, H.; Kim, T.; Hyeon, T.; Jeong, D.H.; Lee, Y.-S. Magnetic field induced aggregation of nanoparticles for sensitive molecular detection. Phys. Chem. Chem. Phys. 2011, 13, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, H.; Kong, L.; Qiao, H.; Li, Y.; Xin, F.; Hao, A. Controlled transformation from nanorods to vesicles induced by cyclomaltoheptaoses (β-cyclodextrins). Carbohydr. Res. 2011, 346, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Provder, T. Challenges in particle size distribution measurement past, present and for the 21st century. Prog. Org. Coat. 1997, 32, 143–153. [Google Scholar] [CrossRef]
- Douliez, J.-P.; Houinsou Houssou, B.; Fameau, A.-L.; Navailles, L.; Nallet, F.; Grélard, A.; Dufourc, E.J.; Gaillard, C. Self-assembly of bilayer vesicles made of saturated long chain fatty acids. Langmuir 2016, 32, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Falvey, P.; Lim, C.W.; Darcy, R.; Revermann, T.; Karst, U.; Giesbers, M.; Marcelis, A.; Lazar, A.; Coleman, A.W.; Reinhoudt, D.N. Bilayer vesicles of amphiphilic cyclodextrins: Host membranes that recognize guest molecules. Chem. Eur. J. 2005, 11, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Vilanova, N.; Solans, C. Vitamin A palmitate-β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Oh, C.; Oh, S.-G. Controlled release of retinol from silica particles prepared in O/W/O emulsion: The effects of surfactants and polymers. J. Control. Release 2005, 106, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; Valiente, M.; Pons, R.; Montalvo, G. Effect of fatty acids on self-assembly of soybean lecithin systems. Colloids Surf. B 2015, 131, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Stevens, R.C. Coupling of an induced fit enzyme to polydiacetylene thin films: Colorimetric detection of glucose. Adv. Mater. 1997, 9, 481–483. [Google Scholar] [CrossRef]
- Cho, E.; Kim, H.; Yang, J.E.; Jun, B.-H.; Paik, S.R.; Jung, S. Supramolecular self-assembled aggregates formed by pentacosa-10,12-diynyl amidomethyl-β-cyclodextrin. Carbohydr. Res. 2014, 391, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Zhang, X.; Sun, S.J.; Sun, G.J.; Yao, K.D.; Liang, D.C.; Guo, G.; Zhang, J.Y. N-alkylated chitosan as a potential nonviral vector for gene transfection. Bioconjug. Chem. 2003, 14, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.






| Kinds of β-CD | Molecular Weight (g/mol) | cac (mM) | Solubility in Water at 25 °C (mg/mL) |
|---|---|---|---|
| β-CD | 1134 | 2.47 | 18.2 |
| S-β-CD | 1423 | 0.09 | 0.6 |
| O-β-CD | 1421 | 0.26 | 1.6 |
| S-β-CD | O-β-CD | |
|---|---|---|
| Initial all-trans-retinol concentration (μmol) | 1.3 | 1.3 |
| Fatty amide-β-CD concentraion (μmol) | 3 | 3 |
| Encapsulation efficiency (%) | 50.18 a | 21.68 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hu, Y.; Jeong, D.; Jun, B.-H.; Cho, E.; Jung, S. Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-cyclodextrin Conjugates. Molecules 2016, 21, 963. https://doi.org/10.3390/molecules21070963
Kim H, Hu Y, Jeong D, Jun B-H, Cho E, Jung S. Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-cyclodextrin Conjugates. Molecules. 2016; 21(7):963. https://doi.org/10.3390/molecules21070963
Chicago/Turabian StyleKim, Hwanhee, Yiluo Hu, Daham Jeong, Bong-Hyun Jun, Eunae Cho, and Seunho Jung. 2016. "Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-cyclodextrin Conjugates" Molecules 21, no. 7: 963. https://doi.org/10.3390/molecules21070963
APA StyleKim, H., Hu, Y., Jeong, D., Jun, B.-H., Cho, E., & Jung, S. (2016). Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-cyclodextrin Conjugates. Molecules, 21(7), 963. https://doi.org/10.3390/molecules21070963