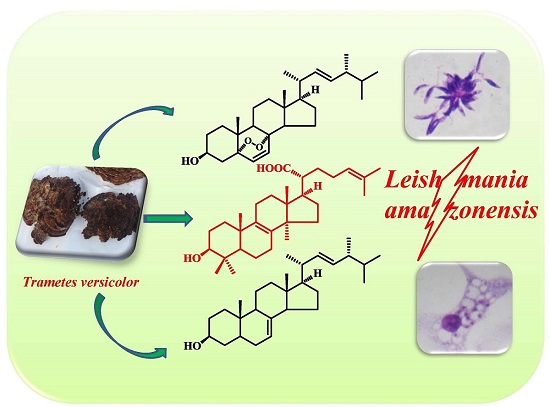
In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden)
Abstract
:1. Introduction
2. Results
2.1. Compound Isolation
2.2. Leishmanicidal Activity
3. Discussion
4. Materials and Methods
4.1. Mushroom Material
4.2. Extract Preparation and Metabolites Isolation
4.3. Structure Elucidation
4.4. Parasites
4.5. Anti-Promastigote Assay
4.6. Anti-Amastigote Activity
4.7. Cytotoxicity Assay
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, N.; Mishra, B.B.; Bajbai, S.; Singh, R.K.; Tiwari, V.K. Natural product based leads to fight against leishmaniasis. Bioorg. Med. Chem. 2014, 22, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; The WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Copeland, N.K.; Aronson, N.E. Leishmaniasis: Treatment updates and clinical practice guidelines review. Curr. Opin. Infect. Dis. 2015, 28, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Rai, M.; Chakravarty, J.; Agarwal, D.; Agrawal, N.; Vaillant, M.; Olliaro, P.; Murray, H.W. New treatment approach in Indian visceral leishmaniasis: Single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin. Infect. Dis. 2008, 47, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.D. Human Leishmaniasis: Clinical, diagnostic and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 1997, 24, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of new drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.S.; Donza, M.R.H.; Rodrigues, A.P.D.; Silva, B.J.M.; Brasil, D.S.B.; Zoghbi, M.G.B.; Andrade, E.H.A.; Guilhon, G.M.S.P.; Silva, E.O. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae). Molecules 2015, 20, 22157–22169. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.M.; Duarte, A.E.; Bezerra Morais-Braga, M.F.; Pansera Waczuk, E.; Vega, C.; Figueiredo Leite, N.; Alencar de Menezes, I.R.; Melo Coutinho, H.D.; Teixeira Rocha, J.B.; Kamdem, J.P. Chemical characterization and trypanocidal, leishmanicidal and cytotoxicity potential of Lantana camara L. (Verbenaceae) essential oil. Molecules 2016, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Roldós, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Singh, R.K.; Srivastava, A.; Tripathi, V.J.; Tiwari, V.K. Fighting against leishmaniasis: Search of alkaloids as future true potential anti-leishmanial agents. Mini-Rev. Med. Chem. 2009, 9, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.G.; Almeida, J.R.G.S.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural products as a source for treatting negleted parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Hadas, E. The use of phytotherapy in diseases caused by parasitic protozoa. Acta Parasitol. 2014, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-J.; Xiao, J.-H. Secondary metabolites from higher fungi: Discovery, bioactivity, bioproduction. Adv. Biochem. Eng. Biotechnol. 2009, 113, 79–150. [Google Scholar] [PubMed]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [PubMed]
- Jordan, K.Z. Biologically active compounds from Aphyllophorales (Polypore) Fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar]
- Tsukagoshi, S.; Hashimoto, Y.; Fujii, G.; Kobayashi, H.; Nomoto, K.; Orita, K. Krestin (PSK). Cancer Treat. Rev. 1984, 2, 131–155. [Google Scholar] [CrossRef]
- Yunoki, S.; Tanaka, N.; Hizuta, A.; Orita, K. Enhancement of antitumour cytotoxicity of hepatic lymphocytes by oral administration of PSK. Int. J. Immunopharmacol. 1994, 16, 123–130. [Google Scholar] [CrossRef]
- Valisolalao, J.; Luu, B.; Ourisson, G. Steroides cytotoxiques de polyporus versicolor. Tetrahedron 1983, 39, 2779–2785. [Google Scholar] [CrossRef]
- Habibi, E.; Sadat-Ebrahimi, S.E.; Mousazadeh, S.A.; Amanzadeh, Y. Mycochemical investigation of the Turkey tail medicinal mushroom Trametes versicolor (Higher basidiomicetes): A potential application of isolated compounds in documented pharmacological studies. Int. J. Med. Mushrooms 2015, 17, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Leliebre-Lara, V.; García Parra, M.; Nogueiras Lima, C.; Monzote Fidalgo, L. Qualitative analysis of an ethanolic extract from Trametes versicolor and biological screening against Leishmania amazonensis. Emir. J. Food Agric. 2015, 27, 592–595. [Google Scholar]
- Shin, Y.; Tamai, Y.; Terazawa, M. Chemical Constituents of Inonotus obliquus IV: Triterpene and steroids from cultured mycelia. Eurasian J. For. Res. 2001, 2, 27–30. [Google Scholar]
- Vazirian, M.; Faramarzi, M.A.; Sadat-Ebrahimi, S.E.; Nomsef Esfahani, H.R.; Samadi, N.; Hosseini, S.A.; Asghari, A.; Mayani, A.; Mousazadeh, A.; Asef, M.R.; et al. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Higher basidiomicetes) and its main compounds. Int. J. Med. Mushrooms 2014, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Deyao, D.; Feng, Z.; Xianhui, C.; Xiuyun, J.; Youjian, F.; Lian-Wen, Q.; Jihong, J. Rapid isolation and purification of inotodiol and trametenolic acid from Inonotus obliquus by High-speed Counter-current chromatography with evaporative light scatting detection. Phytochem. Anal. 2011, 22, 419–423. [Google Scholar]
- Kahols, K.; Kangas, L.; Hiltunen, R. Ergosterol peroxide, an active compound from Inonotus radiatus. Planta Med. 1989, 55, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Millot, M.; Tomasi, S.; Articus, K.; Rouaud, I.; Bernard, A.; Boustie, J. Metabolites from the lichen Ochrolechia parella growing under two different heliotropic conditions. J. Nat. Prod. 2007, 2, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Nakayama, Y.; Yamazaki, M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem. Pharm. Bull. 1994, 42, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Lesnau, A.; Teuscher, E.; Pilgrim, H. Antiviral activity of ergosterol peroxide. Pharmazie 1989, 44, 579–580. [Google Scholar] [PubMed]
- Nakanishi, T.; Murata, H.; Inatomi, Y.; Inada, A.; Murata, J.; Lang, F.A.; Yamasaki, K.; Nakano, M.; Kawahata, T.; Mori, H.; et al. Screening of anti-HIV-1 activity of North American plants. Anti-HIV-1 activities of plant extracts and active components of Lethalia vulpina (L.) Hue. J. Nat. Med. 1998, 52, 521–526. [Google Scholar]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, N.G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 2000, 51, 891–898. [Google Scholar] [CrossRef]
- Ramos-Ligonio, A.; López-Monteon, A.; Trigos, A. Trypanocidal activity of ergosterol peroxide from Pleurotus ostreatus. Phytother. Res. 2012, 26, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Smania, A., Jr.; Delle Monache, F.; Smania, E.F.A.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Horng-Huey, K.; Chi-Feng, H.; Jih-Pyang, W.; Chun-Nan, L. Antiinflammatory triterpenoids and steroids from Ganoderma lucidum and G. tsugae. Phytochemistry 2008, 69, 234–239. [Google Scholar]
- Zhang, Q.; Huang, N.; Wang, J.; Luo, H.; He, H.; Ding, M.; Deng, W.Q.; Zou, K. The H+/K+-ATPase inhibitory activities of Trametenolic acid B from Trametes lactinea (Berk.) Pat, and its effects on gastric cancer cells. Fitoterapia 2013, 89, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, J.A.; Nascimento, E.T.; Monteiro, G.R.; Fernandes, M.Z.; Ramos, A.M.O.; Wilson, M.E.; Pearson, R.D.; Jeronimo, S.M.B. Atypical American visceral leishmaniasis caused by disseminated Leishmania amazonensis infection presenting with hepatitis and adenopathy. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep. 2001, 18, 674–688. [Google Scholar] [PubMed]
- Correa, E.; Cardona, D.; Quiñones, W.; Torres, F.; Franco, A.E.; Vélez, I.D.; Robledo, S.; Echeverri, F. Leishmanicidal activity of Pycnoporus sanguineus. Phytother. Res. 2006, 20, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Goad, L.J.; Holz, G.G., Jr.; Beach, D.H. Sterols of Leishmania species. Implications for biosynthesis. Mol. Biochem. Parasitol. 1984, 10, 161–170. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriés, M.A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Torres-Santos, E.C.; Sampaio-Santos, M.I.; Buckner, F.S.; Yokoyama, K.; Gelb, M.; Urbina, J.A.; Rossi-Bergmann, B. Altered sterol profile induced in Leishmania amazonensis by a natural dihydroxymethoxylated chalcone. J. Antimicrob. Chemother. 2009, 63, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Decock, C.; Herrera, S. Studies in Perenniporia Navisporus ortizii, a synonym of Perenniporia martius, and a note on Navisporus and Perenniporia in Cuba. Cryptogam. Mycol. 2000, 21, 153–162. [Google Scholar] [CrossRef]
- Stamets, P.; Chilton, J.S. The Mushroom Cultivator: A Practical Guide to Growing Mushrooms at Home; Agarikon Press: Olympia, WA, USA, 1983. [Google Scholar]
- Bodley, A.L.; McGarry, M.W.; Shapiro, T.A. Drug cytotoxicity assay for African Trypanosomes and Leishmania species. J. Infect. Dis. 1995, 172, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Caio, T.S.E.; Lima, M.D.; Kaplan, M.A.C.; Nazareth, M.M.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improve MTT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Ueda-Nakamura, T.; Cortez, D.A.G.; Dias Filho, B.P.; Morgado-Díaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 1993, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds N, P and EF are available from the authors.

| Compounds | IC50 a ± SD b | CC50 c ± SD b | SI d | ||||
|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | Macrophages | |||||
| µg/mL | µM | µg/mL | µM | µg/mL | µM | ||
| 1 | 5.9 ± 0.1 | 13.9 ± 0.2 | 1.7 ± 0.1 | 4.0 ± 0.1 | 18.4 ± 0.9 | 42.9 ± 2.2 | 11 |
| 2 | >20 | >50 | >5 | >12 | 14.9 ± 4.9 | 37.5 ± 6.2 | - |
| 3 | 1.3 ± 0.03 | 2.9 ± 0.1 | 0.7 ± 0.03 * | 1.6 ± 0.1 * | 18.0 ± 2.8 | 39.4 ± 4.8 | 25 |
| Pentamidine e | 0.4 ± 0.01 | 1.2 ± 0.02 | 1.3 ± 0.1 | 3.8 ± 0.2 | 11.7 ± 1.7 | 34.4 ± 4.9 | 9 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leliebre-Lara, V.; Monzote Fidalgo, L.; Pferschy-Wenzig, E.-M.; Kunert, O.; Nogueiras Lima, C.; Bauer, R. In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden). Molecules 2016, 21, 1045. https://doi.org/10.3390/molecules21081045
Leliebre-Lara V, Monzote Fidalgo L, Pferschy-Wenzig E-M, Kunert O, Nogueiras Lima C, Bauer R. In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden). Molecules. 2016; 21(8):1045. https://doi.org/10.3390/molecules21081045
Chicago/Turabian StyleLeliebre-Lara, Vivian, Lianet Monzote Fidalgo, Eva-Maria Pferschy-Wenzig, Olaf Kunert, Clara Nogueiras Lima, and Rudolf Bauer. 2016. "In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden)" Molecules 21, no. 8: 1045. https://doi.org/10.3390/molecules21081045