Preparation of Two New Diasteromeric Chiral Stationary Phases Based on (+)-(18-Crown-6)-2,3,11,12-tetracarboxylic Acid and (R)- or (S)-1-(1-Naphthyl)ethylamine and Chiral Tethering Group Effect on the Chiral Recognition
Abstract
:1. Introduction
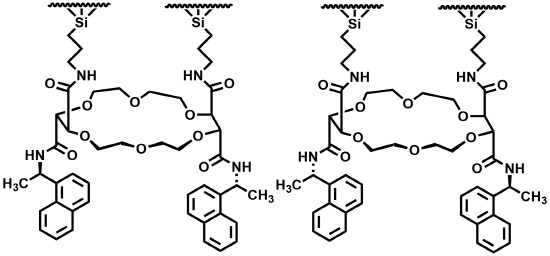
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of CSPs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CSP | Chiral stationary phase |
| EEDQ | 2-Ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline |
| PAA | N-(3,5-Dinitrobenzoyl)-1-phenylalkylamines |
| AAE | N-(3,5-Dinitrobenzoyl)-α-amino acid esters |
| NPA | N-(3,5-Dinitrobenzoyl)-α-amino acid N-propyl amides |
| NNDA | N-(3,5-Dinitrobenzoyl)-α-amino acid N,N-dialkyl amides |
References
- Allenmark, S.G. Chromatographic Enantioseparation: Methods and Applications, 2nd ed.; Ellis Horward: Chichester, UK, 1993; Volume 16, p. 120. [Google Scholar]
- Zhang, Y.; Wu, D.-R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective chromatography in drug discovery. Drug Dis. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Francotte, E. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Andersson, S.; Allenmark, S.G. Preparative chiral chromatographic resolution of enantiomers in drug discovery. J. Biochem. Biophys. Methods 2002, 54, 11–23. [Google Scholar] [CrossRef]
- Francotte, E. Chiral stationary phases for preparative enantioselective chromatography. In Preparative Enantioselectice Chromatography; Cox, G.B., Ed.; Blackwell Publishing: Oxford, UK, 2005; Chapter 3; pp. 48–77. [Google Scholar]
- Chankvetadze, B. Recent developments on polysaccharide–based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ikai, T.; Okamoto, Y. Synthesis and application of immobilized polysaccharide–based chiral stationary phases for enantioseparation by high-performance liquid chromatography. J. Chromatogr. A 2014, 1363, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ng, S.-C.; Tan, T.T.Y.; Wang, Y. Recent development of cyclode×trin chiral stationary phases and their applications in chromatography. J. Chromatogr. A 2012, 1269, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Faris, A.B., III. Chiral separations using the macrocyclic antibiotics: A review. J. Chromatogr. A 2001, 906, 73–89. [Google Scholar] [CrossRef]
- Ilisz, I.; Pataj, Z.; Aranyi, A.; Peter, A. Macrocyclic antibiotic selectors in direct HPLC enantioseparations. Sep. Purif. Rev. 2012, 41, 207–249. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Breitbach, Z.S.; Zhang, Y.; Armstrong, D.W. Development of new HPLC chiral stationary phases based on native and derivatized cyclofructans. Anal. Chem. 2009, 81, 10215–10226. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, A.S.; Lim, Y.; Xu, Q.-L.; Kurti, L.; Armstrong, D.W.; Breitbach, J.S. Enantiomeric separations of α-aryl ketones with cyclofructans chiral stationary phases via high performance liquid chromatography and super critical fluid chromatography. J. Chromatogr. A 2016, 1427, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Cho, H.S.; Hyun, M.H. Liquid chromatographic resolution of 3-amino-1,4-benzodiazepin-2-one derivatives on various Pirkle-type chiral stationary phases. Chirality 2011, 23, E16–E21. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small molecules as chromatographic tools for HPLC enantiomeric resolution: Pirkle-type chiral stationary phases evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Lammerhofer, M. Liquid chromatographic enantiomer separation with special focus on zwitterionic chiral ion-exchangers. Anal. Bioanal. Chem. 2014, 406, 6095–6103. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Sardella, R.; Carotti, A.; Natalini, B.; Lindner, W.; Lammerhofer, M. Quinine-Based Zwitterionic Chiral Stationary Phase as a Complementary Tool for Peptide Analysis: Mobile Phase Effects on Enantio- and Stereoselectivity of Underivatized Oligopeptides. Chirality 2016, 28, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Davankov, V.A. Enantioselective ligand exchange in modern separation techniques. J. Chromatogr. A 2003, 1000, 891–915. [Google Scholar] [CrossRef]
- Ha, J.J.; Han, H.J.; Kim, H.E.; Jin, J.S.; Jeong, E.D. Development of an improved ligand exchange chiral stationary phase based on leucinol for the resolution of proton pump inhibitors. J. Pharm. Biomed. Anal. 2014, 100, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.H. Development and application of crown ether-based HPLC chiral stationary phases. Bull. Korean Chem. Soc. 2005, 26, 1153–1163. [Google Scholar]
- Choi, H.J.; Hyun, M.H. Liquid chromatographic chiral separations by crown ether-based chiral stationary phases. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 853–875. [Google Scholar] [CrossRef]
- Hyun, M.H. Development of HPLC chiral stationary phases based on (+)-(18-crown-6)-2,3,11,12-tetracarboxilic acid and their applications. Chirality 2015, 27, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Masamune, S.; Choy, W.; Petersen, J.S.; Sita, L.R. Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis. Angew. Chem. Int. Ed. 1985, 24, 1–30. [Google Scholar] [CrossRef]
- Hyun, M.H.; Hwang, S.-Y.; Ryoo, J.-J. New chiral stationary phases with two chiral centers for the liquid chromatographic resolution of racemic anti-inflammatory drugs related to α-arylpropionic acids. Chem. Lett. 1994, 23, 1021–1024. [Google Scholar] [CrossRef]
- Cho, Y.J.; Choi, H.J.; Hyun, M.H. Synthesis of new chiral crown ethers incorporating two different chiral units and “matched/mismatched” effect of the two chiral units on the chiral recognition. Bull. Korean Chem. Soc. 2007, 28, 2531–2534. [Google Scholar]
- Kim, H.J.; Choi, H.J.; Cho, Y.J.; Hyun, M.H. Enantiomeric resolution of α-amino acid derivatives on two diastereomeric chiral stationary phases based on chiral crown ethers incorporating two different chiral units. Bull. Korean Chem. Soc. 2010, 31, 1551–1554. [Google Scholar] [CrossRef]
- Behr, J.-P.; Girodeau, J.-M.; Hayward, R.C.; Lehn, J.-M.; Sauvage, J.-P. Molecular receptors. Functionalized and chiral macrocyclic polyethers derived from tartaric acid. Hel. Chim. Acta 1980, 63, 2096–2111. [Google Scholar] [CrossRef]
- Behr, J.-P.; Lehn, J.-M.; Moras, D.; Thierry, J.C. Chiral and functionalized face-discriminated and side-discriminated macrocyclic polyethers. Syntheses and crystal structures. J. Am. Chem. Soc. 1981, 103, 701–703. [Google Scholar] [CrossRef]
- Cross, G.G.; Fyles, T.M. On the synthesis of monoamides of 18-crown-6-tetracarboxylic acid. J. Org. Chem. 1997, 62, 6226–6230. [Google Scholar] [CrossRef]
- Hyun, M.H.; Tan, G.; Xue, J.Y. Unusual resolution of N-(3,5-dinitrobenzoyl)-α-amino acids on a chiral stationary phase based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. J. Chromatogr. A 2005, 1097, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xue, J.Y.; Hyun, M.H. Extended application of a chiral stationary phase based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid to the resolution of N-(substituted benzoyl)-α-amino amides. J. Sep. Sci. 2006, 29, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.H.; Jin, J.S.; Lee, W. Liquid chromatographic resolution of racemic amino acids and their derivatives on a new chiral stationary phase based on crown ether. J. Chromatogr. A 1998, 822, 155–161. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available as chloroform solutions for HPLC injection from authors.




| PAAs | R | CSP 1 | CSP 2 | ||
|---|---|---|---|---|---|
| k1 | α | k1 | α | ||
| PAA-1 | CH3 | 15.98 (S) | 2.19 | 13.78 (R) | 1.95 |
| PAA-2 | n-C5H6 | 12.69 | 1.94 | 12.01 | 1.75 |
| PAA-3 | n-C10H21 | 9.90 | 1.88 | 9.66 | 1.66 |
| PAA-4 | n-C13H27 | 8.34 | 1.86 | 8.93 | 1.66 |
| PAA-5 | n-C15H31 | 8.00 | 1.85 | 8.47 | 1.66 |
| PAA-6 | n-C17H35 | 7.58 | 1.85 | 8.10 | 1.66 |
| AAEs | R1 | R2 | CSP 1 | CSP 2 | ||
|---|---|---|---|---|---|---|
| k1 | α | k1 | α | |||
| AAE-1 | CH3 | CH3 | 10.60 (S) | 1.93 | 12.70 (R) | 1.55 |
| AAE-2 | CH3 | C2H5 | 7.47 (S) | 1.94 | 8.70 (R) | 1.59 |
| AAE-3 | (CH3)2CH | CH3 | 8.70 (S) | 2.32 | 7.44 (R) | 1.89 |
| AAE-4 | (CH3)2CH | C2H5 | 6.50 (S) | 2.37 | 5.57 (R) | 1.98 |
| AAE-5 | C6H5 | CH3 | 27.95 (S) | 1.16 | 30.29 (R) | 1.11 |
| AAE-6 | C6H5 | C3H7 | 16.73 (S) | 1.23 | 17.96 (R) | 1.19 |
| AAE-7 | C6H5 | C4H9 | 14.49 (S) | 1.24 | 15.36 (R) | 1.20 |
| AAE-8 | C6H5 | C8H17 | 10.12 (S) | 1.27 | 12.06 (R) | 1.22 |
| AAE-9 | C6H5CH2 | CH3 | 19.78 (S) | 1.50 | 16.10 (R) | 1.40 |
| AAE-10 | C6H5CH2 | C2H5 | 14.75 (S) | 1.57 | 11.94 (R) | 1.47 |
| AAE-11 | 4-HOC6H5CH2 | C2H5 | 42.36 (S) | 1.70 | 35.93 (R) | 1.59 |
| NPAs | R1 | CSP 1 | CSP 2 | ||
|---|---|---|---|---|---|
| k1 | α | k1 | α | ||
| NPA-1 | CH3 | 5.03 (S) | 1.72 | 5.31 (R) | 1.49 |
| NPA-2 | (CH3)2CH | 2.75 (S) | 2.11 | 2.56 (R) | 1.71 |
| NPA-3 | (CH3)2CH2CH | 2.87 (S) | 1.79 | 2.62 (R) | 1.64 |
| NPA-4 | C6H5 | 8.92 (S) | 1.12 | 8.71 (R) | 1.08 |
| NPA-5 | C6H5CH2 | 6.33 (S) | 1.62 | 5.13 (R) | 1.47 |
| NPA-6 | 4-HOC6H5CH2 | 18.85 (S) | 1.69 | 17.49 (R) | 1.47 |
| NNDAs | R1 | R2 | CSP 1 | CSP 2 | ||
|---|---|---|---|---|---|---|
| k1 | α | k1 | α | |||
| NNDA-1 | CH3 | CH3 | 8.72 (S) | 1.53 | 7.20 (R) | 1.32 |
| NNDA-2 | CH3 | CH2CH3 | 3.32 (S) | 1.61 | 3.29 (R) | 1.41 |
| NNDA-3 | CH3 | CH2CH2CH3 | 2.03 (S) | 1.58 | 1.98 (R) | 1.41 |
| NNDA-4 | (CH3)2CH | CH3 | 3.43 (S) | 1.77 | 2.97 (R) | 1.51 |
| NNDA-5 | (CH3)2CH | CH2CH3 | 2.43 (S) | 1.84 | 2.01 (R) | 1.66 |
| NNDA-6 | (CH3)2CH | CH2CH2CH3 | 1.42 (S) | 1.74 | 1.22 (R) | 1.61 |
| NNDA-7 | (CH3)2CH2CH | CH3 | 3.74 (S) | 1.48 | 3.18 (R) | 1.40 |
| NNDA-8 | (CH3)2CH2CH | CH2CH3 | 2.21 (S) | 1.62 | 1.90 (R) | 1.51 |
| NNDA-9 | (CH3)2CH2CH | CH2CH2CH3 | 1.98 (S) | 1.69 | 1.85 (R) | 1.62 |
| NNDA-10 | C6H5 | CH3 | 8.88 (S) | 1.22 | 8.02 (R) | 1.20 |
| NNDA-11 | C6H5 | CH2CH3 | 5.80 (S) | 1.18 | 4.76 (R) | 1.16 |
| NNDA-12 | C6H5 | CH2CH2CH3 | 5.41 | 1.00 | 5.36 | 1.00 |
| NNDA-13 | C6H5CH2 | CH3 | 6.40 (S) | 1.53 | 5.06 (R) | 1.36 |
| NNDA-14 | C6H5CH2 | CH2CH3 | 3.96 (S) | 1.87 | 3.03 (R) | 1.51 |
| NNDA-15 | C6H5CH2 | CH2CH2CH3 | 3.63 (S) | 1.82 | 3.12 (R) | 1.64 |
| NNDA-16 | 4-HOC6H5CH2 | CH3 | 15.40 (S) | 1.59 | 12.68 (R) | 1.34 |
| NNDA-17 | 4-HOC6H5CH2 | CH2CH3 | 12.01 (S) | 1.93 | 11.02 (R) | 1.49 |
| NNDA-18 | 4-HOC6H5CH2 | CH2CH2CH3 | 10.89 (S) | 1.98 | 10.42 (R) | 1.62 |
| Analytes | CSP 1 | CSP 2 | CSP 3 | |||
|---|---|---|---|---|---|---|
| k1 | α | k1 | α | k1 | α | |
| PPA-1 | 15.98 (S) | 2.19 | 8.53 (R) | 1.98 | 1.90 (S) | 2.08 |
| AAE-1 | 10.60 (S) | 1.93 | 12.70 (R) | 1.55 | 2.72 (S) | 1.76 |
| AAE-3 | 8.70 (S) | 2.32 | 7.44 (R) | 1.89 | 1.61 (S) | 2.14 |
| AAE-11 | 14.75 (S) | 1.57 | 11.94 (R) | 1.47 | 4.36 (S) | 1.54 |
| NPA-1 | 5.03 (S) | 1.72 | 5.31 (R) | 1.49 | 1.37 (S) | 1.51 |
| NPA-2 | 2.75 (S) | 2.11 | 2.56 (R) | 1.71 | 0.80 (S) | 1.79 |
| NPA-3 | 2.87 (S) | 1.79 | 2.62 (R) | 1.64 | 0.65 (S) | 1.51 |
| NNDA-2 | 3.32 (S) | 1.61 | 3.29 (R) | 1.41 | 1.68 (S) | 1.58 |
| NNDA-5 | 2.43 (S) | 1.84 | 2.01 (R) | 1.66 | 0.80 (S) | 1.80 |
| NNDA-8 | 2.21 (S) | 1.62 | 1.90 (R) | 1.51 | 0.78 (S) | 1.53 |
| NNDA-11 | 5.80 (S) | 1.18 | 4.76 (R) | 1.16 | 1.40 (S) | 1.27 |
| NNDA-14 | 3.96 (S) | 1.87 | 3.03 (R) | 1.51 | 1.10 (S) | 1.53 |
| NNDA-17 | 12.01 (S) | 1.93 | 11.02 (R) | 1.49 | 2.46 (S) | 1.47 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agneeswari, R.; Sung, J.Y.; Jo, E.S.; Jeon, H.Y.; Tamilavan, V.; Hyun, M.H. Preparation of Two New Diasteromeric Chiral Stationary Phases Based on (+)-(18-Crown-6)-2,3,11,12-tetracarboxylic Acid and (R)- or (S)-1-(1-Naphthyl)ethylamine and Chiral Tethering Group Effect on the Chiral Recognition. Molecules 2016, 21, 1051. https://doi.org/10.3390/molecules21081051
Agneeswari R, Sung JY, Jo ES, Jeon HY, Tamilavan V, Hyun MH. Preparation of Two New Diasteromeric Chiral Stationary Phases Based on (+)-(18-Crown-6)-2,3,11,12-tetracarboxylic Acid and (R)- or (S)-1-(1-Naphthyl)ethylamine and Chiral Tethering Group Effect on the Chiral Recognition. Molecules. 2016; 21(8):1051. https://doi.org/10.3390/molecules21081051
Chicago/Turabian StyleAgneeswari, Rajalingam, Ji Yeong Sung, Eun Sol Jo, Hee Young Jeon, Vellaiappillai Tamilavan, and Myung Ho Hyun. 2016. "Preparation of Two New Diasteromeric Chiral Stationary Phases Based on (+)-(18-Crown-6)-2,3,11,12-tetracarboxylic Acid and (R)- or (S)-1-(1-Naphthyl)ethylamine and Chiral Tethering Group Effect on the Chiral Recognition" Molecules 21, no. 8: 1051. https://doi.org/10.3390/molecules21081051