Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway
Abstract
:1. Introduction
2. Results
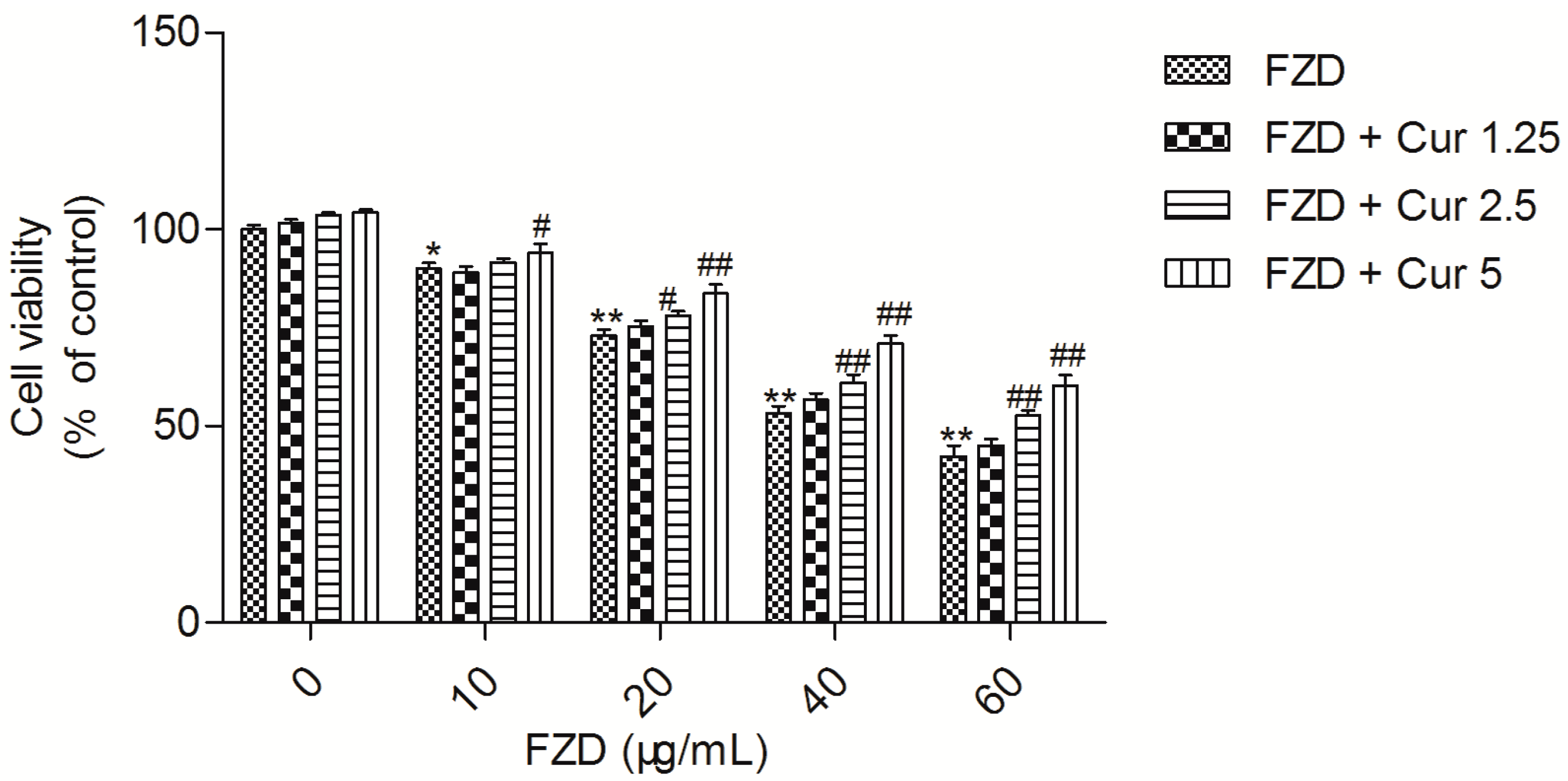
2.1. Curcumin Attenuates FZD Induced Cytotoxicity in L02 Cells
2.2. Curcumin Suppresses FZD Induced Oxidative Stress in L02 Cells
2.3. Curcumin Protects Against FZD Induced the Loss Of Mitochondrial Membrane Potential (Δψm) in L02 Cells
2.4. Curcumin Inhibits FZD Induced the Activations of Caspase-9 and -3
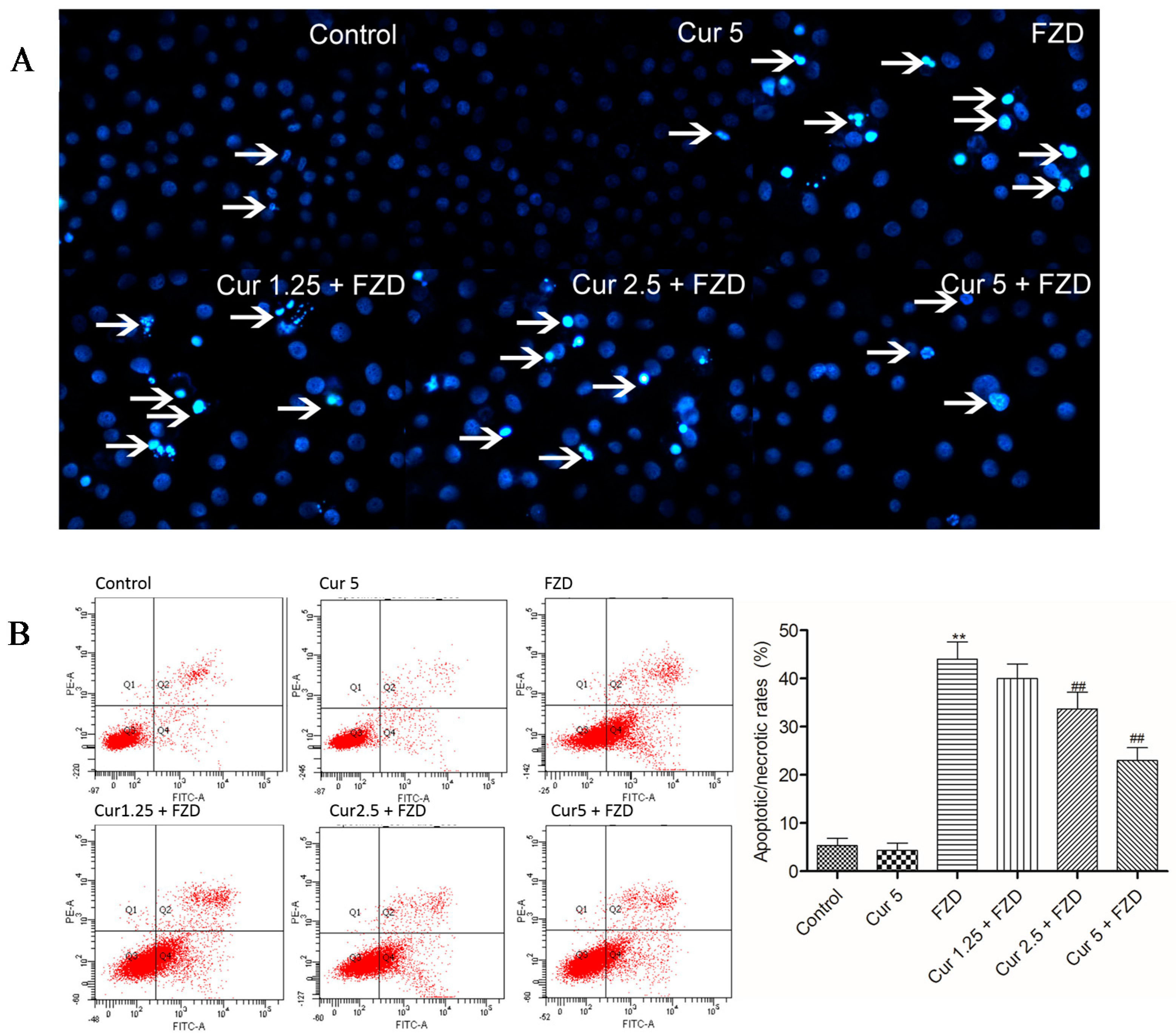
2.5. Curcumin Reduces FZD Induced Apoptosis in L02 Cells
2.6. Curcumin Reduces FZD Induced DNA Damage in L02 Cells
2.7. Curcumin Regulates the mRNA Expression Levels of Apoptosis Related Factors
3. Discussion
4. Materials and Methods
4.1. Chemical and Regents
4.2. Cell Culture
4.3. Measurement of Cell Viability
4.4. Measurement of Intracellular ROS Production
4.5. Measurement of the Activities of SOD, CAT and the Levels of MDA and GSH
4.6. Measurement of Δψm
4.7. Measurement of Caspase-9 and -3 Activities
4.8. Measurement of Apoptosis
4.9. Measurement of DNA Damage by Alkaline Comet Assay
4.10. Measurement of mRNA Expression of Apoptosis Factors by Quantitative Real-Time PCR (qRT-PCR)
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hausen, M.A.; Freitas, J.C.; Monteiro-Leal, L.H. The effects of metronidazole and furazolidone during giardia differentiation into cysts. Exp. Parasitol. 2006, 113, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H. Pharmacological, therapeutic and toxicological properties of furazolidone: Some recent research. Vet. Res. Commun. 1999, 23, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, L.; Qiu, J.J.; Sun, X.; Li, S.; Wang, X.; So, C.W.; Dong, S. A novel application of furazolidone: Anti-leukemic activity in acute myeloid leukemia. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Passos, S.R.; Rodrigues Tde, A.; Madureira, A.P.; Giunchetti, R.C.; Zanini, M.S. Clinical treatment of cutaneous leishmaniasis in dogs with furazolidone and domperidone. Int. J. Antimicrob. Agents 2014, 44, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, Y.; Zhou, H.; Lu, Z.F.; Yang, Z.; Shu, X.; Guo, X.B.; Fan, H.Z.; Tang, J.H.; Zeng, X.P.; et al. Furazolidone-based triple and quadruple eradication therapy for Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 11415–11421. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.F.; Rollinghoff, W.; Preisig, R.; Fisher, M.J. Hepatitis, cardiomyopathy and hemodynamics in furazolidone-induced round heart disease of turkeys. Can. J. Comp. Med. 1979, 43, 345–351. [Google Scholar] [PubMed]
- Gonzalez Borroto, J.I.; Perez Machado, G.; Creus, A.; Marcos, R. Comparative genotoxic evaluation of 2-furylethylenes and 5-nitrofurans by using the comet assay in TK6 cells. Mutagenesis 2005, 20, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Ierardi, E.; Hassan, C.; De Francesco, V. Furazolidone-based therapies for Helicobacter pylori infection: A pooled-data analysis. Saudi J. Gastroenterol. 2012, 18, 11–17. [Google Scholar] [PubMed]
- Jin, X.; Tang, S.; Chen, Q.; Zou, J.; Zhang, T.; Liu, F.; Zhang, S.; Sun, C.; Xiao, X. Furazolidone induced oxidative DNA damage via up-regulating ros that caused cell cycle arrest in human hepatoma HepG2 cells. Toxicol. Lett. 2011, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Wang, Y.; Yang, B.; Ishan, A.; Fang, K.; Peng, D.; Liu, Z.; Dai, M.; Yuan, Z. Tissue depletion and concentration correlations between edible tissues and biological fluids of 3-amino-2-oxazolidinone in pigs fed with a furazolidone-medicated feed. J. Agric. Food Chem. 2010, 58, 6774–6779. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Bujaidar, E.; Ibanez, J.C.; Cassani, M.; Chamorro, G. Effect of furazolidone on sister-chromatid exchanges, cell proliferation kinetics, and mitotic index in vivo and in vitro. J. Toxicol. Environ. Health 1997, 51, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Trabucco, S.E.; Zhang, H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip. Top. Gerontol. 2014, 39, 86–107. [Google Scholar] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Tang, S.; Zhang, S.; Zhang, C.; Wang, C.; Zhou, Y.; Dai, C.; Xiao, X. Furazolidone induces apoptosis through activating reactive oxygen species-dependent mitochondrial signaling pathway and suppressing PI3K/Akt signaling pathway in HepG2 cells. Food Chem. Toxicol. 2015, 75, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Aggarwal, B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Telang, A.G.; Ramya, K.; Vijayakaran, K.; Kesavan, M.; Sarkar, S.N. Protective action of curcumin and nano-Curcumin against arsenic-induced genotoxicity in rats in vivo. Mol. Biol. Rep. 2014, 41, 7413–7422. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Y.; Jia, L.; Jiang, L.P.; Geng, C.Y.; Yao, X.F.; Kong, Y.; Jiang, B.N.; Zhong, L.F. Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J. Agric. Food Chem. 2008, 56, 12059–12063. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Pandey, P.; Tomar, B.; Raisuddin, S.; Parvez, S. Ameliorative action of curcumin in cisplatin-mediated hepatotoxicity: An in vivo study in wistar rats. Arch. Med. Res. 2014, 45, 462–468. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Nose, M.; Ogihara, Y.; Yabu, Y.; Ohta, N. Leishmanicidal effect of curcumin in vitro. Biol. Pharm. Bull. 2002, 25, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Adapala, N.; Chan, M.M. Long-term use of an antiinflammatory, curcumin, suppressed type 1 immunity and exacerbated visceral leishmaniasis in a chronic experimental model. Lab. Investig. 2008, 88, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.B.; Kuttan, R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992, 36, 273–275. [Google Scholar] [PubMed]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In vitro antibacterial activity of curcumin-polymyxin B combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhao, C.; Yan, L.; Qi, R.; Jing, X.; Wang, Z. Sensitizing nanoparticle based platinum (IV) drugs by curcumin for better chemotherapy. Colloids Surf. B Biointerfaces 2016, 145, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.J.; Huang, Y.F.; Chen, X.W.; Luo, Z.T.; Wang, G.X.; Liu, C.C.; Zhang, W.J.; Ouyang, M.Z. Curcumin partly ameliorates irinotecan-induced diarrhea and synergistically promotes apoptosis in colorectal cancer through mediating oxidative stress. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Paget, T.; Elkordy, A.A. Formulation and advantages of furazolidone in liposomal drug delivery systems. Eur. J. Pharm. Sci. 2016, 84, 139–145. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Rossi, L.; Pedersen, J.Z.; Vignoli, A.L.; Vincentini, O.; Hoogenboom, L.A.; Polman, T.H.; Stammati, A.; Zucco, F. Metabolism of furazolidone: Alternative pathways and modes of toxicity in different cell lines. Xenobiotica 1999, 29, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Stammati, A.; Zampaglioni, F.; Zucco, F. Furaltadone cytotoxicity on three cell lines in the presence or absence of DMSO: Comparison with furazolidone. Cell. Biol. Toxicol. 1997, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Ni, Y.C.; Thornton-Manning, J.R.; Fu, P.P.; Heflich, R.H. Mutagenicity of nitrofurantoin and furazolidone in Chinese hamster ovary cell strains. Mutat. Res. 1989, 225, 181–187. [Google Scholar] [CrossRef]
- Stammati, A.; Zampaglioni, F.; Macri, A. Cytotoxic effects of furazolidone on HEp-2 cell line. Ann. Ist. Super Sanità 1987, 23, 165–168. [Google Scholar] [PubMed]
- Ali, B.H.; Hassan, T.; Wasfi, I.A.; Mustafa, A.I. Toxicity of furazolidone to Nubian goats. Vet. Hum. Toxicol. 1984, 26, 197–200. [Google Scholar] [PubMed]
- Zhao, X.C.; Zhang, L.; Yu, H.X.; Sun, Z.; Lin, X.F.; Tan, C.; Lu, R.R. Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem. 2011, 129, 387–394. [Google Scholar] [CrossRef]
- Dai, C.S.; Li, J.C.; Tang, S.S.; Li, J.; Xiao, X.L. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother 2014, 58, 4075–4085. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Gomes, A.C.; Coutinho, O.P. Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur. J. Pharmacol. 2008, 601, 50–60. [Google Scholar] [CrossRef]
- Stroo, W.E.; Schaffer, S.W. Furazolidone-enhanced production of free radicals by avian cardiac and hepatic microsomal membranes. Toxicol. Appl. Pharmacol. 1989, 98, 81–86. [Google Scholar] [CrossRef]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid peroxidation and the thiobarbituric acid assay: Standardization of the assay when using saturated and unsaturated fatty acids. J. Biochem. Mol. Biol. 2004, 37, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F.; Schafer, F.Q.; Buettner, G.R.; Rodgers, V.G. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic Res. 2007, 41, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; McClure, D.; Jimenez, L.A.; Megson, I.L.; Rahman, I. Curcumin induces glutathione biosynthesis and inhibits NF-kappa B activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid. Redox Sign 2005, 7, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Eke, D.; Celik, A. Curcumin prevents perfluorooctane sulfonate-induced genotoxicity and oxidative DNA damage in rat peripheral blood. Drug Chem. Toxicol. 2016, 39, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.S.; Tang, S.S.; Li, D.W.; Zhao, K.N.; Xiao, X.L. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol. Mech. Method 2015, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Rajendra Prasad, N.; Menon, V.P. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. 2006, 611, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Zheng, Z.M.; Zhang, K.B.; Wang, T.P.; Sribastav, S.S.; Liu, W.S.; Liu, T. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis 2011, 16, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Li, J.; Li, J. New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Exp. Toxicol. Pathol. 2013, 65, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Thampithak, A.; Meesarapee, B.; Ratanachamnong, P.; Suksamrarn, A.; Phivthong-Ngam, L.; Phumala-Morales, N.; Chongthammakun, S.; Govitrapong, P.; Sanvarinda, Y. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci. Lett. 2011, 489, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Parvez, S. Mitochondrial dysfunction mediated cisplatin induced toxicity: Modulatory role of curcumin. Food Chem. Toxicol. 2013, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ozaki, T.; Nakanishi, M.; Kikuchi, H.; Yoshida, K.; Horie, H.; Kuwano, H.; Nakagawara, A. Oxidative stress induces p53-dependent apoptosis in hepatoblastoma cell through its nuclear translocation. Genes Cells 2007, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, S.; Jin, X.; Zhang, C.; Zhao, W.; Xiao, X. Involvement of the p38 MAPK signaling pathway in S-phase cell-cycle arrest induced by furazolidone in human hepatoma G2 cells. J. Appl. Toxicol. 2013, 33, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, S.; Xiao, X. The effect of GADD45a on furazolidone-induced S-phase cell-cycle arrest in human hepatoma G2 Cells. J. Biochem. Mol. Toxicol. 2015, 29, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Chen, Q.; Jin, X.; Tang, S.S.; Chen, K.P.; Zhang, T.; Xiao, X.L. Olaquindox induces apoptosis through the mitochondrial pathway in HepG2 cells. Toxicology 2011, 285, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.






| Gene | Primer Sequences (5′–3′) | Product Size (bp) |
|---|---|---|
| Caspase-9 | Forward: 5′-gaggttctcagaccggaaacac-3′ Reverse: 5′-catttcccctcaaactctcaaga-3′ | 90 |
| Caspase-3 | Forward: 5′-gcgaatcaatggactctggaat-3′ Reverse: 5′-aggttgctgcatcgacatctg-3′ | 270 |
| Bax | Forward: 5′-gatgcgtccaccaagaagct-3′ Reverse: 5′-cggccccagttgaagttg-3′ | 169 |
| Bcl-2 | Forward: 5′-gcggagttcacagctctatac-3′ Reverse: 5′-aaaaggcccctacagttacca-3′ | 136 |
| p53 | Forward: 5′-ccctcctcagcatcttatcc-3′ Reverse: 5′-gcacaaacacgcacctcaa-3′ | 260 |
| β-actin | Forward: 5′-gggaaatcgtgcgtgac-3′ Reverse: 5′-ttgccaatggtgatgacctg-3′ | 138 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Li, D.; Gong, L.; Xiao, X.; Tang, S. Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules 2016, 21, 1061. https://doi.org/10.3390/molecules21081061
Dai C, Li D, Gong L, Xiao X, Tang S. Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules. 2016; 21(8):1061. https://doi.org/10.3390/molecules21081061
Chicago/Turabian StyleDai, Chongshan, Daowen Li, Lijing Gong, Xilong Xiao, and Shusheng Tang. 2016. "Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway" Molecules 21, no. 8: 1061. https://doi.org/10.3390/molecules21081061
APA StyleDai, C., Li, D., Gong, L., Xiao, X., & Tang, S. (2016). Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules, 21(8), 1061. https://doi.org/10.3390/molecules21081061





