Rapid Isolation and Determination of Flavones in Biological Samples Using Zinc Complexation Coupled with High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results
2.1. Optimizing Conditions for Flavone Isolation Using Zinc Salt
2.1.1. Recovery of the Isolation Procedure
2.1.2. Concentration Limit of the Separation
2.1.3. Repeatability of the Separation
2.2. Validation of the HPLC Procedure
2.2.1. Selectivity
2.2.2. Linearities, Limits of Detection and Limits of Quantification
2.3. Determination of Baicalin, Baicalein, Wogonoside and Wogonin in Different Parts of S. baicalensis
2.3.1. Effects of Zinc Pretreatment on the Selectivity of the Quantification
2.3.2. Quantification of Scutellarin, Baicalin, Baicalein, Wogonoside and Wogonin
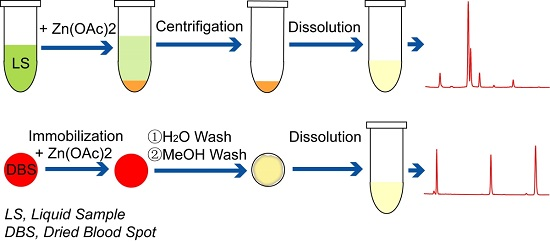
2.4. Determination of Scutellarin, Baicalin, Wogonoside in Artificial Dried Blood Spots
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Isolation of Flavones Using Zinc Acetate
4.3. Isolation of Flavones from Different Parts of S. baicalensis Georgi
4.3.1. Extraction
4.3.2. Isolating Flavones Using Zinc Acetate
4.4. Isolation of Flavones from Dried Blood Spots
4.4.1. Preparation of Artificial Dried Blood Spots
4.4.2. Isolation of Baicalin, Wogonoside and Wogonin from Dried Blood Spots
4.5. Chromatography and Mass Spectrometry
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar] [PubMed]
- Ikemoto, S.; Sugimura, K.; Yoshida, N.; Yasumoto, R.; Wada, S.; Yamamoto, K.; Kishimoto, T. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000, 55, 951–955. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid Baicalin Inhibits HIV-1 Infection at the Level of Viral Entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Rangarajan, P.; Kan, E.M.; Wu, Y.; Wu, C.; Ling, E.-A. Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia. J. Neuroinflamm. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Sheng, Y.; Yang, R.; Lu, B.; Bai, Q.; Ji, L.; Wang, Z. Scutellarin protects against the liver injury induced by diosbulbin B in mice and its mechanism. J. Ethnopharmacol. 2015, 164, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Cole, I.B.; Cao, J.; Alan, A.R.; Saxena, P.K.; Murch, S.J. Comparisons of Scutellaria baicalensis, Scutellaria lateriflora and Scutellaria racemosa: genome size, antioxidant potential and phytochemistry. Plant. Med. 2008, 74, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lian, X.Y.; Li, S.; Stringer, J.L. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine 2009, 16, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Iwabuchi, K.; Mimaki, Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their alpha-glucosidase inhibitory activities. Nat. Prod. Commun. 2012, 7, 471–474. [Google Scholar] [PubMed]
- Wang, D.; Wang, L.; Gu, J.; Yang, H.; Liu, N.; Lin, Y.; Li, X.; Shao, C. Scutellarin Inhibits High Glucose–induced and Hypoxia-mimetic Agent–induced Angiogenic Effects in Human Retinal Endothelial Cells through Reactive Oxygen Species/Hypoxia-inducible Factor-1α/Vascular Endothelial Growth Factor Pathway. J. Cardiovasc. Pharmacol. 2014, 64, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, Y. Determination of baicalin in the leaf of Scutellaria baicalensis with HPLC. Nei. Mongol. J. Tradit. Chin. Med. 2009, 23, 89. [Google Scholar]
- Pryor, J.N.; Bogdanor, J.M.; Welsh, W.A. Process for the Removal of Chlorophyll, Color Bodies and Phospholipids from Glyceride oils Using Acid-Treated Silica Adsorbents. U.S. Patent 4781864 A, 1 November 1988. [Google Scholar]
- Nguyen, T.D.; Han, E.M.; Seo, M.S.; Kim, S.R.; Yun, M.Y.; Lee, D.M.; Lee, G.-H. A multi-residue method for the determination of 203 pesticides in rice paddies using gas chromatography/mass spectrometry. Anal. Chim. Acta 2008, 619, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A.; Hostettmann, M. Preparative Chromatography Techniques: Applications in Nat. Product Isolation, 2nd ed.; Springer: Berlin, Germany, 1998; p. 9. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bijttebier, S.; D’Hondt, E.; Noten, B.; Hermans, N.; Apers, S.; Voorspoels, S. Tackling the challenge of selective analytical clean-up of complex Nat. extracts: The curious case of chlorophyll removal. Food Chem. 2014, 163, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tse, F.L.S. Dried blood spot sampling in combination with LC/MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.T.; Lim, S.-H.; Chan, E.; Ho, P.C. Estimation and comparison of carbamazepine population pharmacokinetics using dried blood spot and plasma concentrations from people with epilepsy: The clinical implication. J. Clin. Pharmacol. 2014, 54, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.V.; Henion, J.; Wickremsinhe, E. Fully-automated approach for online dried blood spot extraction and bioanalysis by two-dimensional-liquid chromatography coupled with high-resolution quadrupole time-of-flight mass spectrometry. Anal. Chem. 2014, 86, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, J.; Wang, Y.; Xiao, S. Simultaneous Determination of Glycyrrhizin and 15 Flavonoids in Licorice and Blood by High Performance Liquid Chromatography with Ultraviolet Detector. ISRN Anal. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.; Xu, S.; Gu, D.; Wang, Y.; Xiao, S. Depletion of high-abundance flavonoids by metal complexation and identification of low-abundance flavonoids in Scutellaria baicalensis Georgi. J. Chromatogr. 2013, 1315, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lapouge, C.; Dangleterre, L.; Cornard, J.P. Spectroscopic and theoretical studies of the Zn(II) chelation with hydroxyflavones. J. Phys. Chem. 2006, 110, 12494–12500. [Google Scholar] [CrossRef] [PubMed]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radical Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, P.H. A fluorometric high-performance liquid chromatography procedure for simultaneous determination of methylamine and aminoacetone in blood and tissues. Anal. Biochem. 2009, 384, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Downey, F.; Ng, C.K.Y. Comparative analysis of bioactive phytochemicals from Scutellaria baicalensis, Scutellaria lateriflora, Scutellaria racemosa, Scutellaria tomentosa and Scutellaria wrightii by LC-DAD-MS. Metabolomics 2011, 446–453. [Google Scholar] [CrossRef]
- Islam, M.N.; Chung, H.J.; Kim, D.H.; Yoo, H.H. A simple isocratic HPLC method for the simultaneous determination of bioactive components of Scutellariae radix extract. Nat. Prod. Res. 2012, 26, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Hishida, A.; Goda, Y.; Mizukami, H. Comparison of the major flavonoid content of S. baicalensis; S. lateriflora; and their commercial products. J. Nat. Med. 2008, 62, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.







| No | k’ | N (m−1) | Rs * | [M − H] − | ||
|---|---|---|---|---|---|---|
| Average | RSD% | Average | RSD% | |||
| 1 | 2.36 | 1.08 | 40,718 | 2.02 | 1.31 ± 0.02 | 463 |
| 2 | 4.00 | 0.91 | 89,299 | 8.44 | 2.08 ± 0.00 | 445 |
| 3 | 6.50 | 1.49 | 54,385 | 8.51 | 3.79 ± 0.03 | 429 |
| 4 | 7.65 | 0.15 | 1,403,517 | 0.26 | 2.77 ± 0.12 | 269 |
| 5 | 8.33 | 0.10 | 1,290,340 | 5.08 | 2.87 ± 0.13 | 373 |
| Compound | a | b | R2 | LOD (ng·inj−1) | LOQ (ng·inj−1) | Range (ng·inj−1) |
|---|---|---|---|---|---|---|
| Scutellarin | 0.0500 ± 0.0000 | −0.5452 ± 0.2750 | 0.9999 | 2.50 | 4.99 | 4.99–499.00 |
| Baicalin | 0.0727 ± 0.0000 | −0.8635 ± 0.0406 | 0.9999 | 2.36 | 4.72 | 4.72–472.00 |
| Wogonoside | 0.1096 ± 0.0001 | 0.0663 ± 0.0022 | 1.0000 | 1.33 | 5.30 | 5.30–530.00 |
| Baicalein | 0.1201 ± 0.0000 | −0.8634 ± 0.0884 | 0.9999 | 1.55 | 3.10 | 3.10–310.00 |
| Wogonin | 0.1775 ± 0.0001 | 0.0668 ± 0.0192 | 1.0000 | 1.65 | 3.30 | 3.30–330.00 |
| Part | Scutellarin | Baicalin | Wogonoside | Baicalein | Wogonin |
|---|---|---|---|---|---|
| Pod | 0.44 ± 0.05 | 0.11 ± 0.03 | 0.02 ± 0.00 | 0.01 ± 0.00 | ND |
| Corolla | 0.78 ± 0.09 | 0.27 ± 0.01 | ND | ND | ND |
| Leaf | 0.91 ± 0.11 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | ND |
| Stem | 0.37 ± 0.04 | 0.05 ± 0.01 | 0.02 ± 0.00 | ND | ND |
| Hierbaculum | 0.15 ± 0.02 | 5.73 ± 0.14 | 0.93 ± 0.00 | 0.65 ± 0.06 | 0.47 ± 0.15 |
| Tap root | 0.14 ± 0.02 | 14.38 ± 0.12 | 2.08 ± 0.00 | 1.79 ± 0.16 | 0.43 ± 0.15 |
| Lateral root | 0.16 ± 0.02 | 12.09 ± 0.30 | 1.73 ± 0.00 | 1.72 ± 0.15 | 0.42 ± 0.15 |
| Root bark | ND | 1.04 ± 0.03 | 0.38 ± 0.00 | 3.71 ± 0.32 | 1.33 ± 0.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Wang, H.; Wang, Y.; Xiao, S. Rapid Isolation and Determination of Flavones in Biological Samples Using Zinc Complexation Coupled with High-Performance Liquid Chromatography. Molecules 2016, 21, 1067. https://doi.org/10.3390/molecules21081067
Sun C, Wang H, Wang Y, Xiao S. Rapid Isolation and Determination of Flavones in Biological Samples Using Zinc Complexation Coupled with High-Performance Liquid Chromatography. Molecules. 2016; 21(8):1067. https://doi.org/10.3390/molecules21081067
Chicago/Turabian StyleSun, Chenghe, Hecheng Wang, Yingping Wang, and Shengyuan Xiao. 2016. "Rapid Isolation and Determination of Flavones in Biological Samples Using Zinc Complexation Coupled with High-Performance Liquid Chromatography" Molecules 21, no. 8: 1067. https://doi.org/10.3390/molecules21081067