Comprehensive Quantitative Analysis of SQ Injection Using Multiple Chromatographic Technologies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of Constituents
2.2. Limitation of Qualitative Analysis Solely Using Mass Spectrometry
2.3. Method Validation
2.4. Quantification of Eighteen Analytes in Commercial Shenqi Fuzheng Injection (SQI) Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample Preparation
3.3. Ultra-Performance Liquid-Chromatography Tandem Mass Spectrometry (UPLC-MS) Conditions
3.4. HPLC-NH2P-ELSD Conditions
3.5. High Performance Gel Permeation Chromatography (HPGPC) Conditions
3.6. Acid Hydrolysis
3.7. Method Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwok, K.Y.; Xu, J.; Ho, H.M.; Chen, H.B.; Li, M.; Lang, Y.; Han, Q.B. Quality evaluation of commercial Huang-Lian-Jie-Du-Tang based on simultaneous determination of fourteen major chemical constituents using high performance liquid chromatography. J. Pharm. Biomed. Anal. 2013, 85, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.B.; Yue, R.Q.; Xu, J.; Ho, H.M.; Ma, D.L.; Leung, C.H.; Chau, S.L.; Zhao, Z.Z.; Chen, H.B.; Han, Q.B. Comprehensive quantitative analysis of Shuang-Huang-Lian oral liquid using UHPLC-Q-TOF-MS and HPLC-ELSD. J. Pharm. Biomed. Anal. 2015, 102. [Google Scholar] [CrossRef] [PubMed]
- Pan, L. Practical road of “the numeral turn Chinese herbal medicine” for shenqi fuzheng injection. J. China Prescript Drug 2009, 1, 37–39. [Google Scholar]
- Li, H. Efficacy of shenqi fuzheng injection in adjuvant therapy of intractable heart failure. Chin. J. Geront. 2012, 32, 4982–4983. [Google Scholar]
- Yang, J.Q.; Li, Y.; Zhao, Q.; Kuang, G.; Fan, L.; Wang, L.; Zhang, H.; Jiao, K.; Zhou, H. Effect of shenqi fuzheng injection on pre/post-operational change of argyrophilic-nucleolar organizer regions in peripheral T-lymphocyte in patients with gastric carcinoma. Chin. J. Intergr. Tradit. West Med. 2005, 25, 626–628. [Google Scholar]
- Zhao, Q.; Li, Y.; Wang, L.L. Effect of shenqi fuzheng injection on immune function in gastric carcinoma patients in post-operational and chemotherapeutic period. Chin. J. Intergr. Tradit. West Med. 2001, 21, 424–426. [Google Scholar]
- Zhang, Y.; Guo, L.L.; Zhao, S.P. Effect of shenqi fuzheng injection combined with chemotherapy in treating colorectal cancer. Chin. J. Intergr. Tradit. West Med. 2010, 30, 280–282. [Google Scholar]
- Lin, H.S.; Li, D.R. Multi-center randomized clinical study on Shenqi Fuzheng injection combined with chemotherapy in the treatment for lung cancer. Chin. J. Oncol. 2007, 29, 931–934. [Google Scholar]
- Huang, Z.F.; Wei, J.S.; Li, H.Z. Effect of shenqi fuzheng injection combined with chemotherapy on thirty patients with advanced breast cancer. Chin. J. Intergr. Tradit. West Med. 2008, 28, 152–154. [Google Scholar]
- Bo, Y.; Li, H.S.; Qi, Y.C.; Lu, M.Y. Clinical study on treatment of mammary cancer by shenqi fuzheng injection in cooperation with chemotherapy. Chin. J. Integr. Med. 2007, 13, 37–40. [Google Scholar] [PubMed]
- Zhu, X.Y.; Zhang, X.Z.; Zhong, X.Y. Effect of shenqi fuzheng injection for hemopoietic and immune function reconstruction in patients with hematologic malignancies undergoing chemotherapy. Chin. J. Intergr. Tradit. West Med. 2010, 30, 205–207. [Google Scholar]
- Li, H.S.; Yang, B.; Su, X.C. Effect of shenqi fuzheng injection on repairing the immune function in patients with breast cancer. Chin. J. Intergr. Tradit. West Med. 2009, 29, 537–539. [Google Scholar]
- Liang, Q.L.; Pan, D.C.; Xie, J.R. Effect of shenqi fuzheng injection combined with chemotherapy in treating advanced colorectal carcinoma. Chin. J. Intergr. Tradit. West Med. 2009, 29, 439–441. [Google Scholar]
- Dai, Z.; Wan, X.; Kang, H.; Ji, Z.; Liu, L.; Liu, X.; Song, L.; Min, W.; Ma, X. Clinical effects of shenqi fuzheng injection in the neoadjuvant chemotherapy for local advanced breast cancer and the effects on T-lymphocyte subsets. J. Tradit. Chin. Med. 2008, 28, 34–38. [Google Scholar] [PubMed]
- Zhao, J.M.; Wu, A.Z.; Shi, L.R. Clinical observation on treatment of advanced gastric cancer by combined use of shenqi fuzheng injection, docetaxel, flurouracil and calcium folinate. Chin. J. Intergr. Tradit. West Med. 2007, 27, 736–738. [Google Scholar]
- Li, Z.; Liu, Z.; Hou, X.; Qian, Q.; Liu, S. Protective effect and mechanism of shenqi fuzheng injection on diabetic glomerulopathy in rats with streptozotocin-induced diabetes. Chin. J. Hosp. Pharm. 2002, 22, 15–17. [Google Scholar]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of shenqi fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharm. 2012, 139, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.R.; Wang, J.N.; Liu, L.; Chen, X.; Chen, M.S.; Chen, J.; Ren, J.H.; Li, Q.; Han, J. Modulation of radiation-induced tumour necrosis factor-alpha and transforming growth factor beta1 expression in the lung tissue by shengqi fuzheng injection. Mol. Med. Rep. 2010, 3, 621–627. [Google Scholar] [PubMed]
- Chen, J.; Wang, Z.; Wu, T. Shenqi fuzheng injection improves CVB3-induced myocarditis via inhibiting TRAF6 expression. Cell Mol. Biol. 2013, 59, 1826–1834. [Google Scholar]
- Song, J.Z.; Yiu, H.W.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Chemical comparison and classification of Radix astragali by determination of isoflavonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.F.; He, Z.D.; Han, Q.B.; Xu, H.X.; Jiang, R.W.; Li, S.L.; Zhang, Y.B.; But, P.P.H.; Shaw, P.C. The use of lobetyolin and HPLC-UV fingerprints for quality assessment of Radix codonopsis. J. Food Drug Anal. 2007, 15, 258–264. [Google Scholar]
- Napolitano, A.; Akay, S.; Mari, A.; Bedir, E.; Pizza, C.; Piacente, S. An analytical approach based on ESI-MS, LC-MS and PCA for the quali-quantitative analysis of cycloartane derivatives in Astragalus spp. J. Pharm. Biomed. Anal. 2013, 85, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, T.S.; Hu, G.; Fang, Y.Z. Determination of sugars in Chinese traditional drugs by CE with amperometric detection. J. Pharm. Biomed. Anal. 2002, 30, 1047–1053. [Google Scholar] [CrossRef]
- Lin, L.C.; Tsai, T.H.; Kuo, C.L. Chemical constituents comparison of Codonopsis tangshen, Codonopsis pilosula var. modesta and Codonopsis pilosula. Nat. Prod. Res. 2013, 27, 1812–1815. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Tong, X.; Wang, J.X.; Zou, W.; Cao, H.; Su, W.W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula shenqi fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, J.A.; Jeon, H.J.; Kim, S.; Kim, Y.H.; Kim, H.Y.; Whang, W.K. Chemical fingerprinting of Codonopsis pilosula and simultaneous analysis of its major components by HPLC-UV. Arch. Pharm. Res. 2014, 37, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, C.; Wang, L.; Liu, X.; Sun, X.; Ye, W. Chemical constituents of shenqi fuzheng injection. Chin. Tradit. Pat. Med. 2011, 33, 1743–1747. [Google Scholar]
- Xu, J.; Chen, H.B.; Liu, J.; Kwok, K.Y.; Yue, R.Q.; Yi, T.; Ho, H.M.; Zhao, Z.Z.; Han, Q.B. Why are Angelicae Sinensis radix and Chuanxiong Rhizoma different? An explanation from a chemical perspective. Food Res. Internat. 2013, 54, 439–447. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.L.; Yue, R.Q.; Ko, C.H.; Hu, J.M.; Liu, J.; Ho, H.M.; Yi, T.; Zhao, Z.Z.; Zhou, J.; et al. A novel and rapid HPGPC-based strategy for quality control of saccharide-dominant herbal materials: Dendrobium officinale, a case study. Anal. Bioanal. Chem. 2014, 406, 6409–6417. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yue, R.Q.; Liu, J.; Ho, H.M.; Yi, T.; Chen, H.B.; Han, Q.B. Structural diversity requires individual optimization of ethanol concentration in precipitation of natural polysaccharides. Int. J. Biol. Macromol. 2014, 67, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Chan, B.C.L.; Yu, H.; Yang, Y.H.; Hu, S.Q.; Ko, C.H.; Dong, C.X.; Wong, C.K.; Shaw, P.C.; Fung, K.P.; et al. Structural characterization and immuno-modulating activities of a polysaccharide from Ganoderma sinense. Internat. J. Biol. Macromol. 2012, 51, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Livzon Pharm. Group Inc. A Immunomodulating Composition of Radix Codonopsis and Radix Astragali and its Preparation. CN 1686404A; S.I.P.O.o. T.P.R.C, 26 October 2005. [Google Scholar]
- Liu, X.; Tu, F.; Tao, D. Quality Control of shenqi fuzheng Injections Containing Radix codonopsis and Astragalus. CN1899362A; S.I.P.O.o. T.P.R.C, 24 January 2007. [Google Scholar]
- Pharmacopoeia Commission of P.R. China. Formal Approved standard (s) for New Drugs; People′s Medical Publishing House: Beijing, China, 2003; pp. 50–59. [Google Scholar]
- Li, F.; Cheng, T.F.; Dong, X.; Li, P.; Yang, H. Global analysis of chemical constituents in shengmai injection using high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 117, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, L.; Gao, W.; Liu, K.; Qi, L.W.; Li, P. Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography-mass spectrometric techniques. J. Pharm. Biomed. Anal. 2014, 92, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from authors.
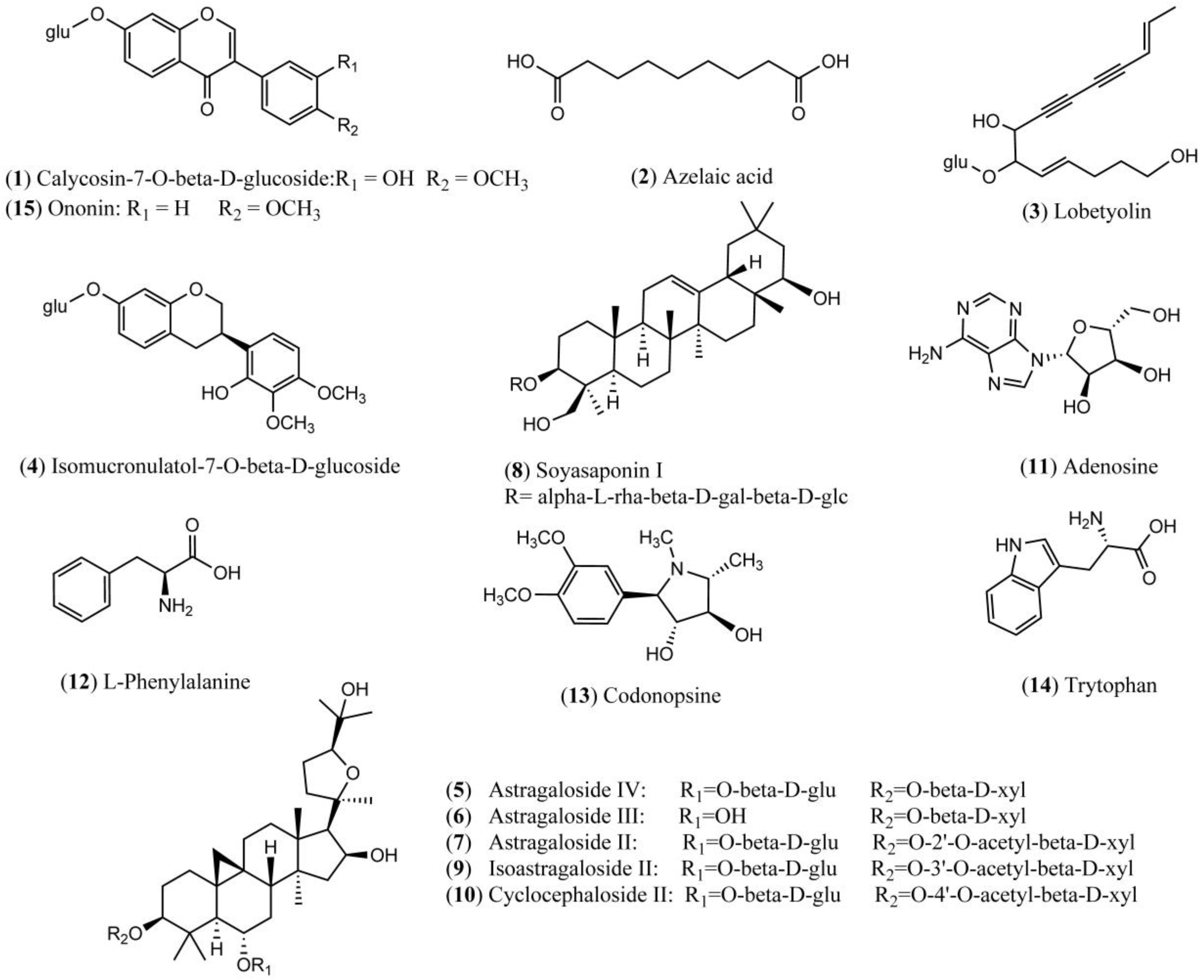
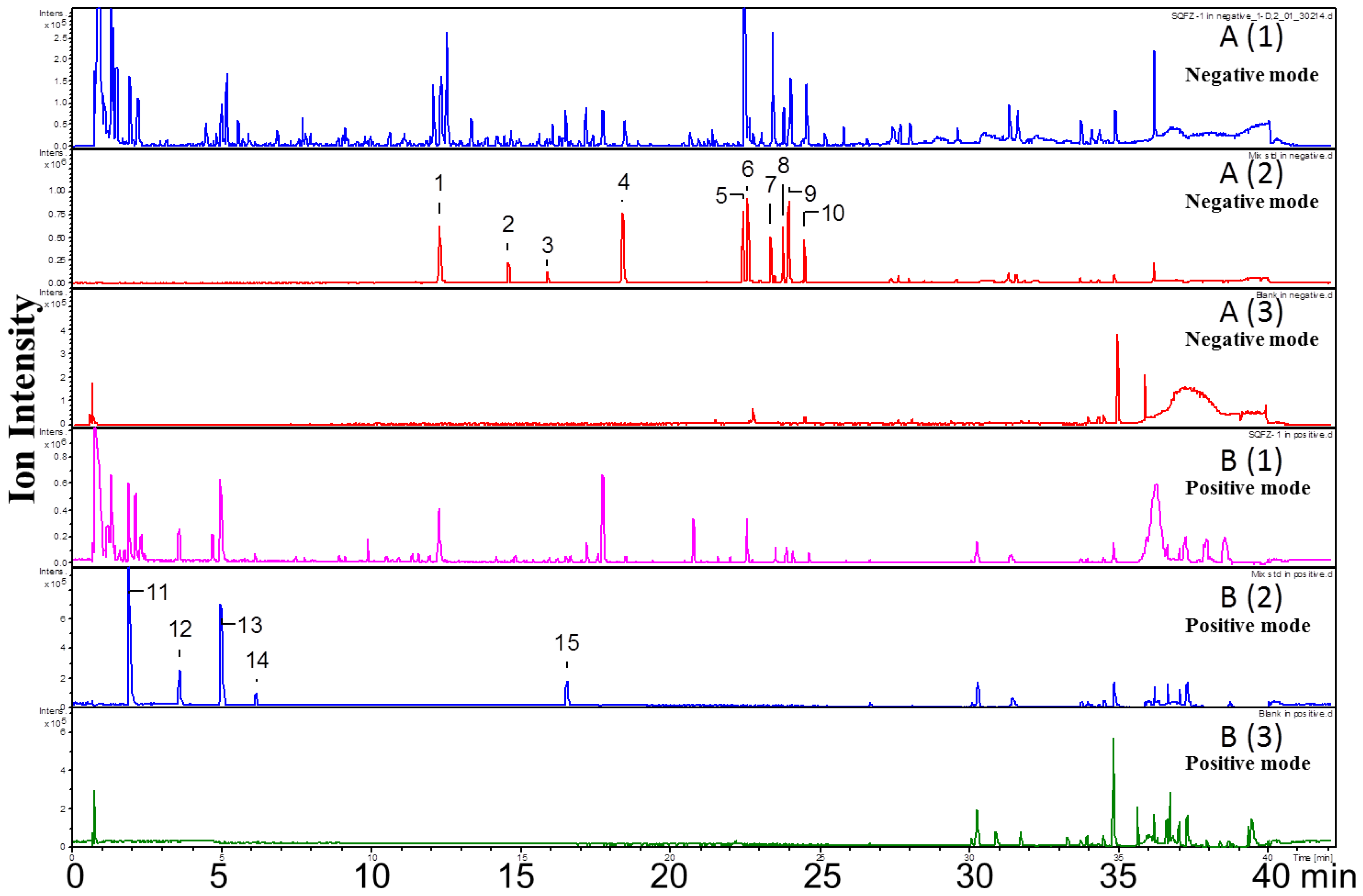

| No. | RT (Min) | Formula | [M + H]+ (Error, ppm) | [M − H]− (Error, ppm) | Fragment Ions in Positive Mode | Fragment Ions in Negative Mode | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 12.3 | C22H22O10 | 445.1150 (3.59) | 491.1215 [M + COOH]−, 481.0918 [M + Cl]−, 283.0623 [M − H − glc]−, 268.0375 [M − H − glc − H2O]−, 224.1435, 184.0517 | Calycosin-7-O-beta-d-glucoside | ||
| 2 | 14.9 | C9H16O4 | 187.0976 (3.21) | 169.0868, 125.0972 | Azelaic acid | ||
| 3 | 16.6 | C20H28O8 | 395.1728 (5.82) | 441.1773 [M + COOH]−, 431.1484 [M + Cl]−, 395.1701, 305.0586, 185.0970, 159.0812, 143.0708, 119.0351, 101.0243 | Lobetyolin | ||
| 4 | 19.2 | C23H28O10 | 463.1619 (3.24) | 509.1671 [M + COOH]−, 499.1383 [M + Cl]−, 345.9224, 301.1080, 254.0532, 135.0443 | Isomucronulatol-7-O-glucoside | ||
| 5 | 23.7 | C41H68O14 | 785.4671 (2.04) | 783.4554 (3.06) | (829.4593 [M + COOH]−, 819.4307 [M + Cl]−, 707.2920, 651.4105, 577.6834, 490.3592, 357.5564, 279.2332, 179.0567, 161.0450, 131.0343, 119.0352, 113.0245, 101.0246) | Astragaloside IV | |
| 6 | 23.8 | C41H68O14 | 783.4538 (1.02) | (829.4593 [M + COOH]−, 819.4297 [M + Cl]−, 621.4005, 489.3581, 394.8312, 279.2329, 161.0464, 113.0251, 101.0243) | Astragaloside III | ||
| 7 | 24.7 | C43H70O15 | 825.4628 (0.97) | (871.4710 [M + COOH]−, 861.4408 [M + Cl]−, 765.4429, 719.7998, 520.4337, 338.1722, 224.1057, 179.0572, 143.0355, 119.0348, 101.0252) | Astragaloside II | ||
| 8 | 25.2 | C48H78O18 | 943.5269 (0.32) | 941.5130 (2.23) | (879.5089, 733.4564, 615.3915, 457.3659, 362.4478, 247.0827, 163.0604) | Soyasaponin I | |
| 9 | 25.4 | C43H70O15 | 825.4659 (2.79) | (871.4702 [M + COOH]−, 861.4404 [M + Cl]−, 726.3011, 593.3695, 465.2882, 336.2658, 257.2371, 179.0583, 113.0243) | Isoastragaloside II | ||
| 10 | 26.0 | C43H70O15 | 825.4618 (2.18) | (871.4705 [M + COOH]−, 861.4405 [M + Cl]−, 765.4444, 603.3852, 335.0594, 223.8866, 179.0537, 161.0435, 143.0337, 113.0220) | Cyclocephaloside II | ||
| 11 | 1.8 | C10H13N5O4 | 268.1059 (5.22) | 211.9763, 178.0760, 136.0622, 119.0350 | Adenosine | ||
| 12 | 3.4 | C9H11NO2 | 166.0863 (3.01) | l-Phenylalanine | |||
| 13 | 5.1 | C14H21NO4 | 268.1556 (2.98) | 220.1272, 161.0537, 118.0810, 100.0709 | Codonopsine | ||
| 14 | 6.0 | C11H12N2O2 | [M + H − OH]+ 188.0943, (3.19) | Trytophan | |||
| 15 | 17.1 | C22H22O9 | 431.1343 (0.23) | 453.1158 [M + Na]+, 350.0444, 269.0813, 213.0919, 118.0416 | Ononin |
| Analyte | EIC Ions | Linearity | LOD (µg/mL) | LOQ (µg/mL) | Repeatability RSD (%) (n = 6) | Stability | Spike Recovery (RSD %) (n = 3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression Equation | R2 | Range (µg/mL) | Intra-Day | Inter-Day | RSD (%) (n = 6) | High | Middle | Low | ||||
| 1 | 283.07 | y = 85.094x − 47097 | 0.9997 | 3.04–12.16 | 0.15 | 0.49 | 1.61 | 3.74 | 3.12 | 101.00 (0.31) | 104.61 (2.25) | 95.32 (2.72) |
| 2 | 187.10 | y = 35.078x + 13967 | 0.9987 | 0.33–2.63 | 0.090 | 0.30 | 2.08 | 4.81 | 4.37 | 99.18 (3.01) | 99.56 (5.04) | 105.53 (1.83) |
| 3 | 441.18 | y = 31.521x − 15298 | 0.9981 | 1.22–3.80 | 0.14 | 0.47 | 2.03 | 2.27 | 3.18 | 97.11 (0.69) | 103.34 (2.45) | 96.33 (3.17) |
| 4 | 463.17 | y = 128.05x − 23035 | 0.9995 | 0.69–2.75 | 0.11 | 0.34 | 2.68 | 3.18 | 3.33 | 105.38 (0.71) | 104.63 (0.13) | 106.30 (0.53) |
| 5 | 829.47 | y = 595.32x + 39240 | 0.9992 | 0.50–4.00 | 0.0077 | 0.026 | 2.49 | 2.25 | 4.54 | 97.49 (0.80) | 105.62 (0.63) | 95.34 (2.88) |
| 6 | 829.47 | y = 453.52x − 9380.8 | 0.9991 | 0.19–0.93 | 0.0069 | 0.024 | 3.48 | 4.15 | 4.73 | 104.78 (1.25) | 101.74 (3.98) | 102.36 (3.40) |
| 7 | 871.48 | y = 410.39x − 220599 | 0.9990 | 0.57–2.85 | 0.11 | 0.36 | 1.32 | 1.33 | 1.48 | 100.62 (3.05) | 105.35 (1.31) | 95.35 (3.54) |
| 8 | 941.52 | y = 869.66x + 26058 | 0.9995 | 0.26–1.04 | 0.012 | 0.039 | 2.64 | 4.62 | 4.71 | 105.91 (5.70) | 106.59 (2.95) | 96.80 (3.58) |
| 9 | 871.48 | y = 393.52x + 9798.5 | 0.9992 | 0.52–2.60 | 0.14 | 0.49 | 1.69 | 2.90 | 3.88 | 94.97 (3.52) | 98.60 (2.60) | 105.16 (1.80) |
| 10 | 871.48 | y = 338.05x + 14751 | 0.9996 | 0.36–2.92 | 0.0048 | 0.016 | 1.59 | 2.39 | 2.75 | 102.57 (2.63) | 95.38 (3.06) | 96.58 (2.17) |
| 11 | 268.11 | y = 253.34x + 192461 | 0.9992 | 1.46–7.31 | 0.048 | 0.16 | 1.33 | 2.36 | 2.21 | 98.42 (1.79) | 99.91 (0.37) | 101.91 (1.91) |
| 12 | 120.08 | y = 159.12x + 104220 | 0.9991 | 1.48–7.38 | 0.102 | 0.34 | 1.38 | 2.45 | 2.16 | 98.82 (0.59) | 99.90 (3.16) | 98.75 (2.20) |
| 13 | 268.16 | y = 567.08x + 484891 | 0.9996 | 2.42–9.69 | 0.025 | 0.080 | 1.99 | 1.53 | 1.51 | 99.46 (0.68) | 99.75 (0.57) | 97.07 (6.77) |
| 14 | 188.09 | y = 1043.3x − 30639 | 0.9994 | 0.20–0.79 | 0.029 | 0.090 | 3.47 | 4.27 | 3.89 | 100.34 (0.94) | 101.60 (1.20) | 103.81 (4.25) |
| 15 | 431.14 | y = 125.32x − 18966 | 0.9996 | 0.87–2.24 | 0.17 | 0.56 | 3.74 | 4.33 | 4.31 | 103.98 (3.11) | 99.77 (3.71) | 105.11 (0.82) |
| Analyte | SFI-1 | SFI-2 | SFI-3 | SFI-4 | SFI-5 | SFI-6 | SFI-7 | SFI-8 | SFI-9 | SFI-10 | SFI-11 | SFI-12 | SFI-13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.53 | 7.60 | 7.52 | 7.60 | 7.69 | 7.90 | 7.25 | 7.22 | 7.46 | 7.45 | 5.67 | 5.83 | 5.63 |
| 2 | 0.36 | 0.42 | 0.43 | 0.38 | 0.41 | 0.44 | 0.38 | 0.35 | 0.38 | 0.32 | 1.04 | 1.28 | 1.27 |
| 3 | 2.48 | 2.71 | 2.79 | 2.76 | 2.66 | 2.63 | 2.58 | 2.16 | 2.50 | 2.48 | 1.94 | 2.81 | 2.46 |
| 4 | 1.80 | 1.89 | 1.86 | 1.86 | 1.91 | 1.91 | 1.89 | 1.84 | 1.85 | 1.80 | 1.64 | 1.86 | 1.84 |
| 5 | 2.61 | 2.95 | 2.81 | 2.90 | 2.83 | 2.88 | 2.83 | 2.83 | 2.85 | 2.83 | 2.12 | 2.32 | 2.29 |
| 6 | 0.44 | 0.49 | 0.45 | 0.47 | 0.46 | 0.45 | 0.46 | 0.45 | 0.45 | 0.45 | 0.34 | 0.31 | 0.31 |
| 7 | 2.26 | 2.61 | 2.43 | 2.50 | 2.50 | 2.51 | 2.48 | 2.48 | 2.49 | 2.52 | 1.98 | 1.91 | 1.90 |
| 8 | 1.31 | 1.37 | 1.40 | 1.38 | 1.39 | 1.28 | 1.35 | 1.38 | 1.31 | 1.33 | 0.93 | 1.06 | 1.04 |
| 9 | 0.97 | 1.21 | 1.10 | 1.12 | 1.10 | 1.12 | 1.14 | 1.08 | 1.13 | 1.14 | 0.77 | 0.78 | 0.72 |
| 10 | 1.12 | 1.44 | 1.30 | 1.31 | 1.30 | 1.30 | 1.26 | 1.32 | 1.38 | 1.37 | 0.94 | 0.95 | 0.87 |
| 11 | 7.39 | 7.82 | 7.92 | 7.64 | 7.42 | 7.23 | 7.38 | 7.31 | 8.04 | 8.18 | 7.06 | 8.48 | 8.60 |
| 12 | 5.52 | 5.82 | 5.77 | 5.70 | 5.74 | 5.47 | 5.36 | 5.46 | 5.15 | 5.55 | 5.49 | 5.32 | 5.25 |
| 13 | 5.10 | 4.99 | 5.02 | 4.78 | 4.79 | 4.84 | 5.16 | 4.89 | 4.93 | 4.92 | 6.10 | 4.93 | 4.87 |
| 14 | 0.33 | 0.30 | 0.27 | 0.36 | 0.31 | 0.29 | 0.30 | 0.37 | 0.26 | 0.26 | 0.19 | 0.31 | 0.43 |
| 15 | 1.92 | 1.82 | 1.73 | 1.77 | 1.86 | 1.71 | 1.32 | 1.63 | 1.85 | 1.76 | 1.17 | 1.18 | 1.15 |
| Sub-total | 40.56 | 42.94 | 42.20 | 41.99 | 41.79 | 41.52 | 40.65 | 40.26 | 41.59 | 43.00 | 37.06 | 38.93 | 38.23 |
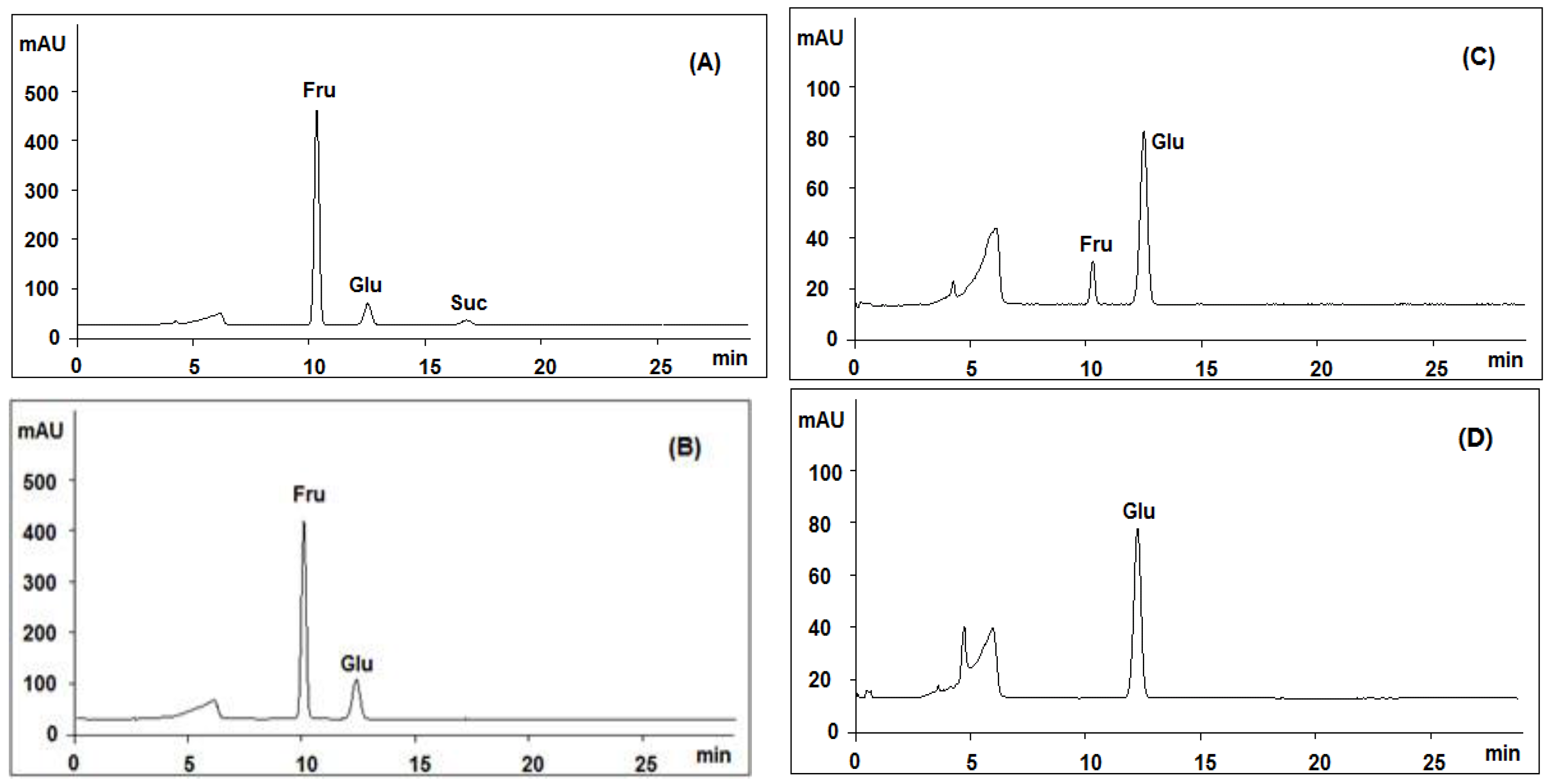
| Fructose a | 7.13 | 7.10 | 7.14 | 7.14 | 7.14 | 7.17 | 7.15 | 7.17 | 7.18 | 7.63 | 7.49 | 7.41 | 7.13 |
| Glucose a | 2.72 | 2.73 | 2.73 | 2.84 | 2.82 | 2.76 | 2.75 | 2.78 | 2.73 | 3.04 | 2.76 | 2.74 | 2.72 |
| Sucrose a | 1.33 | 1.33 | 1.33 | 1.37 | 1.33 | 1.34 | 1.35 | 1.36 | 1.34 | 1.22 | 1.23 | 1.21 | 1.33 |
| Salt a,b | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 | 7.2 |
| Dry weight a | 19.91 | 20.39 | 19.91 | 20.16 | 19.57 | 19.79 | 19.59 | 20.12 | 20.02 | 20.43 | 20.97 | 20.59 | 20.9 |
| Content percentage | 92.52 | 90.25 | 92.63 | 92.22 | 94.69 | 93.54 | 94.39 | 92.20 | 92.37 | 93.65 | 89.26 | 90.33 | 88.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, S.-L.; Huang, Z.-B.; Song, Y.-G.; Yue, R.-Q.; Ho, A.; Lin, C.-Z.; Huang, W.-H.; Han, Q.-B. Comprehensive Quantitative Analysis of SQ Injection Using Multiple Chromatographic Technologies. Molecules 2016, 21, 1092. https://doi.org/10.3390/molecules21081092
Chau S-L, Huang Z-B, Song Y-G, Yue R-Q, Ho A, Lin C-Z, Huang W-H, Han Q-B. Comprehensive Quantitative Analysis of SQ Injection Using Multiple Chromatographic Technologies. Molecules. 2016; 21(8):1092. https://doi.org/10.3390/molecules21081092
Chicago/Turabian StyleChau, Siu-Leung, Zhi-Bing Huang, Yan-Gang Song, Rui-Qi Yue, Alan Ho, Chao-Zhan Lin, Wen-Hua Huang, and Quan-Bin Han. 2016. "Comprehensive Quantitative Analysis of SQ Injection Using Multiple Chromatographic Technologies" Molecules 21, no. 8: 1092. https://doi.org/10.3390/molecules21081092






