Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction
Abstract
:1. Introduction
2. Results and Discussion
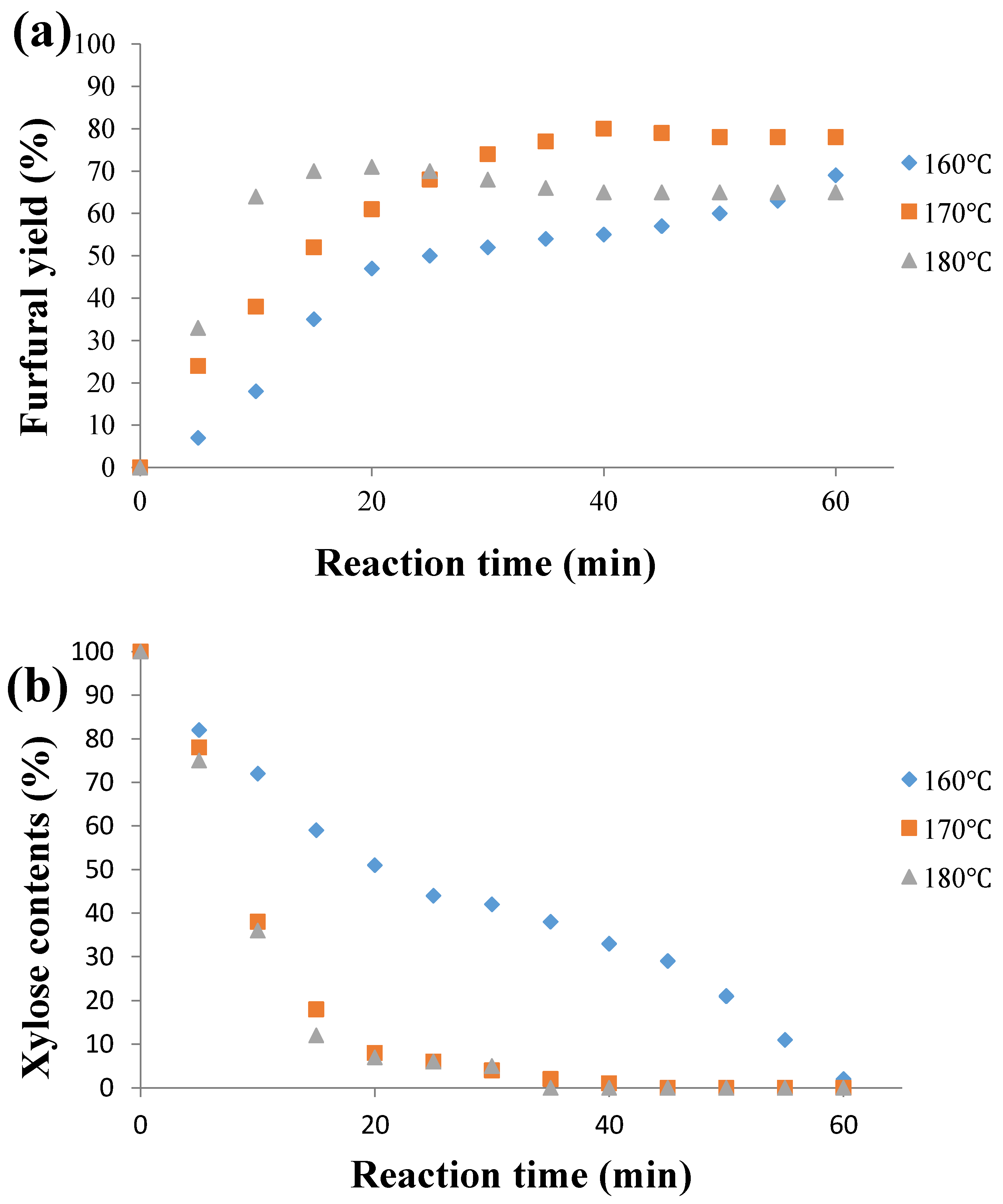
2.1. Influence of the Temperature on Furfural Yield
2.2. Effect of d-xylose Loading Variation
2.3. Influence of the Residence Time on the Furfural Production
2.4. Effect of the Ratio Water-CPME on the Furfural Yield
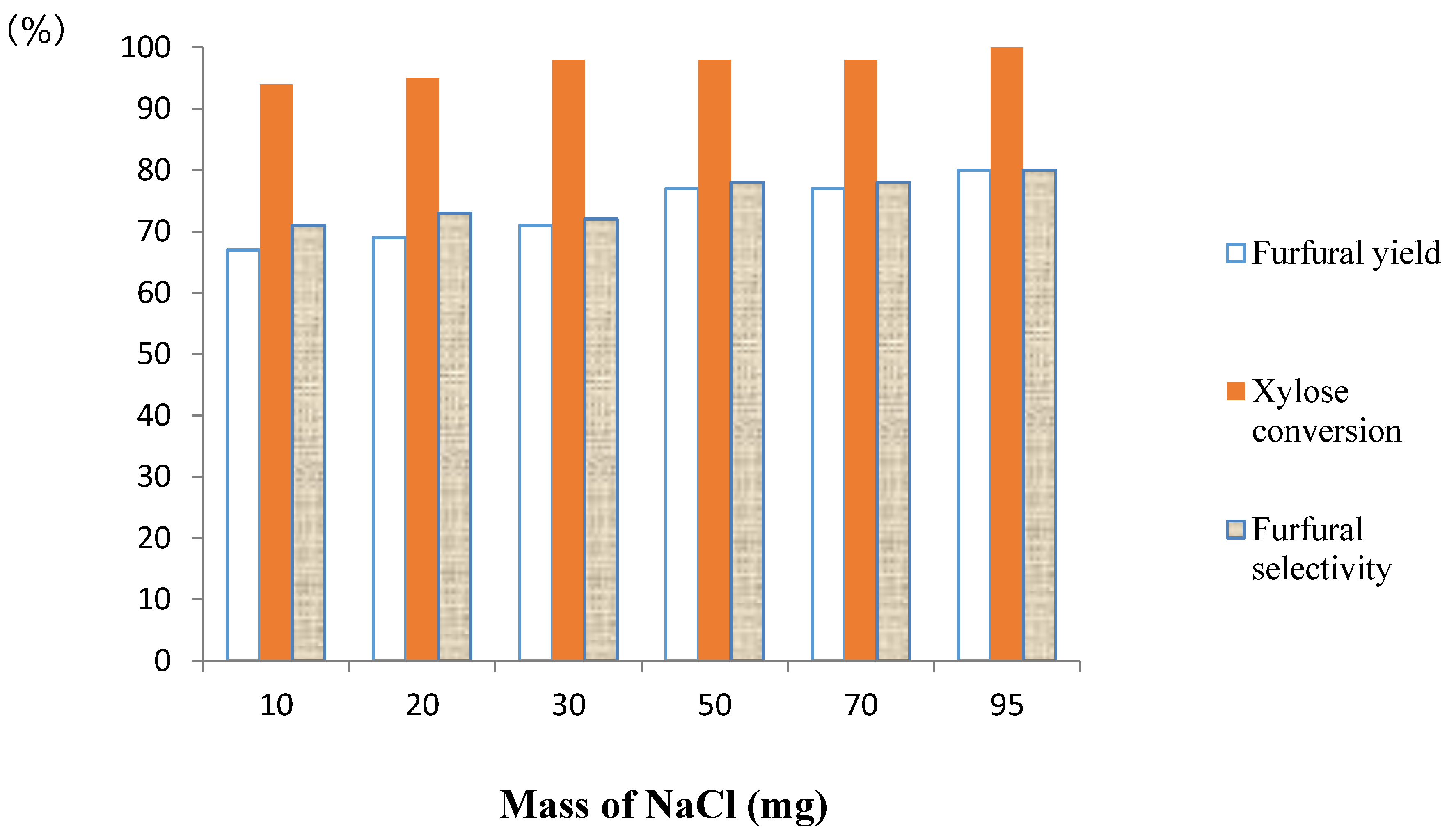
2.5. Influence of the Ratio Nafion NR50-NaCl on the Furfural Yield
2.6. Reusability of the Nafion NR50
2.7. SEM Observations EDX and Analysis of Nafion NR50 Pellets
2.8. Furfural Production from Xylan
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Synthesis of Furfural in a Water-CPME Biphasic Media
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPME | cyclopentyl methyl ether |
| DMSO | dimethylsulfoxide |
| EDX | energy dispersive X-ray |
| ELSD | evaporative light scattering detector |
| HPLC | high performance liquid chromatography |
| IR | infrared |
| MeTHF | methyltetrahydrofuran |
| MIBK | methyl isobutyl ketone |
| MW | microwave |
| PFA | polyfurfuryl alcohol |
| PTFE | polytetrafluoroethylene |
| rpm | revolutions per minute |
| SEM | scanning electron microscopy |
| THF | tetrahydrofuran |
References
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2000; Volume 16, pp. 285–313. [Google Scholar]
- Kamireddy, S.R.; Kozliak, E.I.; Tucker, M.; Ji, Y. Kinetic features of xylan de-polymerization in production of xylose monomer and furfural during acid pretreatment for kenaf, foragesorghums and sunn hemp feedstocks. Int. Agric. Biol. Eng. 2014, 7, 86–98. [Google Scholar]
- Marcotullio, G.; De Jong, W. Chloride ions enhance furfural formation from d-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. [Google Scholar] [CrossRef]
- Fulmer, E.I.; Christensen, L.M.; Hixon, R.M.; Foster, R.L. The production of furfural from xylose solutions by means of hydrochloric acid-sodium chloride systems. J. Phys. Chem. 1936, 40, 133–141. [Google Scholar] [CrossRef]
- Rong, C.; Ding, X.; Zhu, Y.; Li, Y.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acide and inorganic salts. Carbohydr. Res. 2012, 350, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Yemis, O.; Mazza, G. Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 2011, 102, 7371–7378. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, J.; Ohara, M.; Nishimura, S.; Ebitani, K. One-pot formation of furfural from xylose via isomerization and successive dehydration reactions over heterogeneous acid and base catalysts. Chem. Lett. 2010, 39, 838–840. [Google Scholar] [CrossRef]
- Agirrezahal-Telleria, I.; Larreategui, A.; Requies, J.; Guemez, M.B.; Arias, P.L. Furfural production from xylose using sulfonic ion-exchange resins (Amberlyst) and simultaneous stripping with nitrogen. Bioresour. Technol. 2011, 102, 7478–7485. [Google Scholar] [CrossRef] [PubMed]
- Aellig, C.; Scholz, D.; Dapsens, P.Y.; Mondelli, C.; Perez Ramirez, J. When catalyst meets reactor: Continuous biphasic processing of xylan to furfural over GaUSY/Amberlyst-36. Catal. Sci. Technol. 2015, 5, 142–149. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, D.W.; Hwang, Y.K.; Lee, V.H.; Hong, D.Y.; Chang, J.S. An integrated process for the production of 2,5-dimethylfuran from fructose. Green Chem. 2015, 17, 3310–3313. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate, niobate and titanoniobate nanosheets as solid acid catalysts for the liquid-phase dehydration of d-xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Tureja, J.; Nishimura, S.; Ebitani, K. One-pot synthesis of furans from various saccharides using combination of solid acid and base catalysts. Bull. Chem. Soc. Jpn. 2012, 85, 275–281. [Google Scholar]
- Moreau, C.; Durand, R.; Peyron, D.; Duhamet, J.; Rivalier, P. Selective preparation of furfural from xylose over microporous solid acid catalysts. Ind. Crop Prod. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr. Res. 2006, 341, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Hajid, E.; Leung, A.C.W.; Chong, J.H.; Hahnoud, K.A.; Luong, J.H.T. Synthesis of furfural from xylose by heterogeneous and reusable Nafion catalysts. ChemSusChem 2011, 4, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Dwiatmoko, A.A.; Choi, J.W.; Suh, Y.W.; Suh, D.J.; Oh, M. Cellulose pretreatment with 1-n-butyl-3-methylimidazolium chloride for solid acid-catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 8273–8279. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl methyl ether as a new and alternative process solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Conner, W.C., Jr.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.; Bo, D.; Chang, H.; Wang, X.; Zhu, T. Optimization of furfural production from d-xylose with formic acid as catalyst in a reactive extraction system. Bioresour. Technol. 2013, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Karimi, K.; Roodpeyma, S. Production of furans from rice straw by single-phase and biphasic systems. Carbohydr. Res. 2010, 345, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived monoa and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C., Jr.; Huber, G.W. Design of solid acid catalysts for aqueous phase dehydration of carbohydrates. The role of Lewis and Bronsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Le Guenic, S.; Delbecq, F.; Ceballos, C.; Len, C. Microwave-assisted dehydration of d-xylose into furfural by diluted inexpensive inorganic salts solution in a biphasic system. J. Mol. Catal. A Chem. 2015, 410, 1–7. [Google Scholar] [CrossRef]
- Lopez, D.E.; Goodwin, J.G., Jr.; Bruce, D.A. Transesterification of triacetin with methanol on Nafion acid resins. J. Catal. 2007, 245, 381–391. [Google Scholar] [CrossRef]
- Danon, B.; Hongsiri, W.; van der Aa, L.; de Jong, W. Kinetic study on homogeneous catalyzed xylose dehydration to furfural in the presence of arabinose and glucose. Biomass Bioenergy 2014, 66, 364–370. [Google Scholar] [CrossRef]
- Kootstra, A.M.J.; Mosier, N.S.; Scott, E.L.; Beeftink, H.H.; Sanders, J.P.M. Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochem. Eng. J. 2009, 43, 92–97. [Google Scholar] [CrossRef]
- Gairola, K.; Smirnova, I. Hydrothermal pentose to furfural conversion and simultaneous extraction with SC-CO2 kinetics and application to biomass hydrolysates. Bioresour. Technol. 2012, 123, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Dvorchik, B.H. Kinetics and mechanisms of the acid degradation of the aldopentose to furfural. J. Pharm. Sci. 1969, 58, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.S.J.; Verweij, M.D.; van Gerven, T.; Stankiewicz, A.I.; Staefanidis, G.D. On the effect of resonant microwave fields on temperature distribution in time and space. Int. J. Heat Mass Transf. 2012, 55, 3800–3811. [Google Scholar] [CrossRef]
- Xiouras, C.; Radacsi, N.; Sturm, G.; Stefanidis, G.D. Furfural synthesis in the presence of sodium chloride: Microwaves versus conventional heating. ChemSusChem 2016. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Guenic, S.; Gergela, D.; Ceballos, C.; Delbecq, F.; Len, C. Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction. Molecules 2016, 21, 1102. https://doi.org/10.3390/molecules21081102
Le Guenic S, Gergela D, Ceballos C, Delbecq F, Len C. Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction. Molecules. 2016; 21(8):1102. https://doi.org/10.3390/molecules21081102
Chicago/Turabian StyleLe Guenic, Sarah, David Gergela, Claire Ceballos, Frederic Delbecq, and Christophe Len. 2016. "Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction" Molecules 21, no. 8: 1102. https://doi.org/10.3390/molecules21081102
APA StyleLe Guenic, S., Gergela, D., Ceballos, C., Delbecq, F., & Len, C. (2016). Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction. Molecules, 21(8), 1102. https://doi.org/10.3390/molecules21081102