N,N′-Bis(2-cyclohexylethyl)naphtho[2,3-b:6,7-b′]dithiophene Diimides: Effects of Substituents
Abstract
:1. Introduction
2. Results
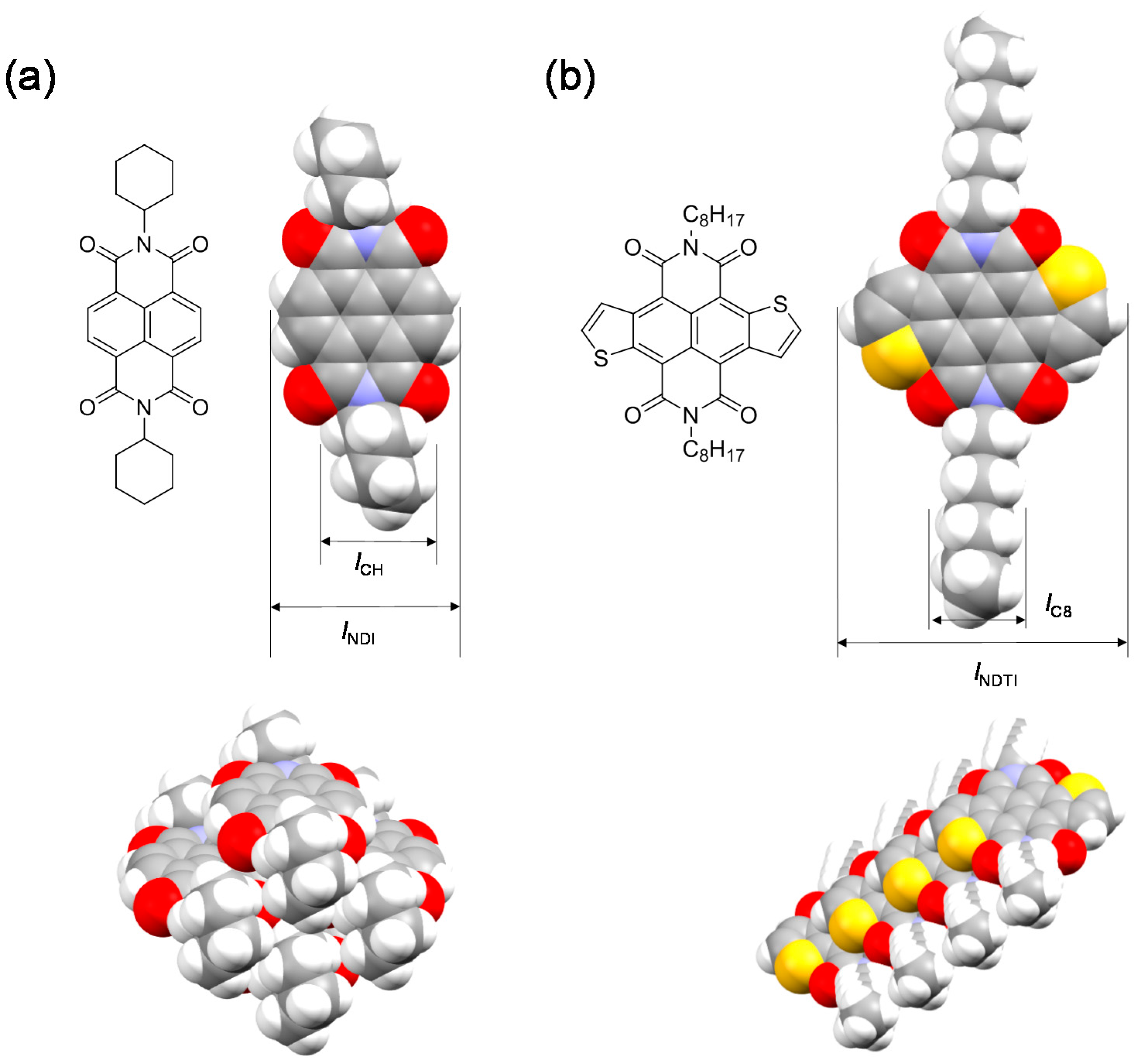
2.1. Molecular Design and Synthesis
2.2. Physicochemical Properties
2.3. Single-Crystal Structures
2.4. Thin-Film Deposition and Field-Effect Transistors
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Single-Crystal X-ray Analysis
4.3. Device Fabrication and Evaluation
4.4. Theoretical Calculations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Würthner, F.; Ahmed, S.; Thalacker, C.; Debaerdemaeker, T. Core-substituted naphthalene bisimides: New fluorophors with tunable emission wavelength for FRET studies. Chem. Eur. J. 2002, 8, 4742–4750. [Google Scholar] [CrossRef]
- Sakai, N.; Mareda, J.; Vauthey, E.; Matile, S. Core-substituted naphthalenediimides. Chem. Commun. 2010, 46, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.-L.; Würthner, F. Strategies for the synthesis of functional naphthalene diimides. Angew. Chem. Inter. Ed. 2014, 53, 7428–7448. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Jani, C.H.; Langford, S.V. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.; Mullen, K. Beyond perylene diimides: Synthesis, assembly and function of higher rylene chromophores. J. Mater. Chem. C 2014, 2, 1938–1956. [Google Scholar] [CrossRef]
- Langhals, H.; Zgela, D.; Lüling, R. Sexterrylenetetracarboxylic Bisimides: NIR Dyes. J. Org. Chem. 2015, 80, 12146–12150. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, S.; Tanaka, K.; Maruya, Y.; Mori, S.; Masuo, S.; Okujima, T.; Uno, H.; Nakayama, K.-I.; Yamada, H. Synthesis of pentacene-, tetracene- and anthracene bisimides using double-cyclization reaction mediated by bismuth(III) triflate. Chem. Commun. 2011, 47, 10112–10114. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Gao, J.; Li, Y.; Jiang, W.; Di Motta, S.; Negri, F.; Wang, Z. One-Pot Synthesis of Stable NIR Tetracene Diimides via Double Cross-Coupling. J. Am. Chem. Soc. 2011, 133, 18054–18057. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Lv, A.; Gao, J.; Jiang, W.; Hao, L.; Li, C.; Li, Y.; Polander, L.E.; Barlow, S.; Hu, W.; et al. Hybrid Rylene Arrays via Combination of Stille Coupling and C–H Transformation as High-Performance Electron Transport Materials. J. Am. Chem. Soc. 2012, 134, 5770–5773. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, X.; Di, C.-A.; Yang, X.; Zhang, F.; Liu, Y.; Li, H.; Zhu, D. Core-Expanded Naphthalene Diimides Fused with Sulfur Heterocycles and End-Capped with Electron-Withdrawing Groups for Air-Stable Solution-Processed n-Channel Organic Thin Film Transistors. Chem. Mater. 2011, 23, 1204–1215. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Tan, L.; Yang, G.; Li, Y.; Zhang, G.; Liu, Z.; Xu, W.; Zhang, D. Dithiazole-fused naphthalene diimides toward new n-type semiconductors. J. Mater. Chem. C 2013, 1, 1087–1092. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wang, Z. Synthesis and Properties of Naphthobisbenzothiophene Diimides. Org. Lett. 2013, 15, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiao, C.; Li, Y.; Wang, Z. Synthesis and Properties of Heterocyclic Acene Diimides. Org. Lett. 2013, 15, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chang, J.; Huang, K.-W.; Shi, X.; Wu, J.; Chi, C. Cyanated Diazatetracene Diimides with Ultrahigh Electron Affinity for n-Channel Field Effect Transistors. Org. Lett. 2013, 15, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.-L.; Zschieschang, U.; Klauk, H.; Wurthner, F. A core-extended naphthalene diimide as a p-channel semiconductor. Chem. Commun. 2011, 47, 11504–11506. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, Y.; Nakano, M.; Hu, J.-Y.; Osaka, I.; Takimiya, K. Naphthodithiophenediimide (NDTI): Synthesis, structure, and applications. J. Am. Chem. Soc. 2013, 135, 11445–11448. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Osaka, I.; Hashizume, D.; Takimiya, K. α-Modified Naphthodithiophene Diimides—Molecular Design Strategy for Air-Stable n-Channel Organic Semiconductors. Chem. Mater. 2015, 27, 6418–6425. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Nakano, M.; Osaka, I.; Takimiya, K. Naphthodithiophenediimide (NDTI)-based triads for high-performance air-stable, solution-processed ambipolar organic field-effect transistors. J. Mater. Chem. C 2015, 3, 4244–4249. [Google Scholar] [CrossRef]
- Nakano, M.; Osaka, I.; Takimiya, K. Naphthodithiophene Diimide (NDTI)-Based Semiconducting Copolymers: From Ambipolar to Unipolar n-Type Polymers. Macromolecules 2015, 48, 576–584. [Google Scholar] [CrossRef]
- Zhou, E.; Nakano, M.; Izawa, S.; Cong, J.; Osaka, I.; Takimiya, K.; Tajima, K. All-Polymer Solar Cell with High Near-Infrared Response Based on a Naphthodithiophene Diimide (NDTI) Copolymer. ACS Macro Lett. 2014, 3, 872–875. [Google Scholar] [CrossRef]
- Nakano, K.; Nakano, M.; Xiao, B.; Zhou, E.; Suzuki, K.; Osaka, I.; Takimiya, K.; Tajima, K. Naphthodithiophene Diimide-Based Copolymers: Ambipolar Semiconductors in Field-Effect Transistors and Electron Acceptors with Near-Infrared Response in Polymer Blend Solar Cells. Macromolecules 2016, 49, 1752–1760. [Google Scholar]
- Shukla, D.; Nelson, S.F.; Freeman, D.C.; Rajeswaran, M.; Ahearn, W.G.; Meyer, D.M.; Carey, J.T. Thin-Film Morphology Control in Naphthalene-Diimide-Based Semiconductors: High Mobility n-Type Semiconductor for Organic Thin-Film Transistors. Chem. Mater. 2008, 20, 7486–7491. [Google Scholar] [CrossRef]
- Kakinuma, T.; Kojima, H.; Ashizawa, M.; Matsumoto, H.; Mori, T. Correlation of mobility and molecular packing in organic transistors based on cycloalkyl naphthalene diimides. J. Mater. Chem. C 2013, 1, 5395–5401. [Google Scholar] [CrossRef]
- Payne, M.M.; Parkin, S.R.; Anthony, J.E.; Kuo, C.-C.; Jackson, T.N. Organic Field-Effect Transistors from Solution-Deposited Functionalized Acenes with Mobilities as High as 1 cm2/V·s. J. Am. Chem. Soc. 2005, 127, 4986–4987. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Nakano, M.; Sugino, H.; Osaka, I. Design and elaboration of organic molecules for high field-effect-mobility semiconductors. Synth. Met. 2016, 217, 68–78. [Google Scholar] [CrossRef]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.D.M.; Yap, S.; Jani, C.H.; Bhosale, S.V.; Hofkens, J.; DeSchryver, F.C.; Langford, S.J.; Ghiggino, K.P. Synthesis and Photophysics of Core-Substituted Naphthalene Diimides: Fluorophores for Single Molecule Applications. Chem. Asian J. 2009, 4, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning Orbital Energetics in Arylene Diimide Semiconductors. Materials Design for Ambient Stability of n-Type Charge Transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef] [PubMed]
- ADF: Powerful DFT code for modeling molecules; Scientific Computing and Modeling: Amsterdam. Available online: http://www.scm.com/ADF/ (accessed on 29 July 2016).
- Bondi, A. Van der waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Dawson, R.E.; Hennig, A.; Weimann, D.P.; Emery, D.; Ravikumar, V.; Montenegro, J.; Takeuchi, T.; Gabutti, S.; Mayor, M.; Mareda, J.; et al. Experimental evidence for the functional relevance of anion-π interactions. Nat. Chem. 2010, 2, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHLXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Kunugi, Y. 2,7-Diphenyl[1]benzothieno[3,2-b]benzothiophene, A New Organic Semiconductor for Air-Stable Organic Field-Effect Transistors with Mobilities up to 2.0 cm2·V−1·s−1. J. Am. Chem. Soc. 2006, 128, 12604–12605. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.






| Compound | Eredonset/V 1 | ELUMO/eV 2 | λonset/nm | Eg/eV 3 |
|---|---|---|---|---|
| 3 | −0.35 | −4.0 | 574 | 2.16 |
| 4 | −0.27 | −4.1 | 556 | 2.23 |
| 1 | −0.37 | −4.0 | 569 | 2.17 |
| 2 | −0.28 | −4.1 | 552 | 2.24 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, M.; Hashizume, D.; Takimiya, K. N,N′-Bis(2-cyclohexylethyl)naphtho[2,3-b:6,7-b′]dithiophene Diimides: Effects of Substituents. Molecules 2016, 21, 981. https://doi.org/10.3390/molecules21080981
Nakano M, Hashizume D, Takimiya K. N,N′-Bis(2-cyclohexylethyl)naphtho[2,3-b:6,7-b′]dithiophene Diimides: Effects of Substituents. Molecules. 2016; 21(8):981. https://doi.org/10.3390/molecules21080981
Chicago/Turabian StyleNakano, Masahiro, Daisuke Hashizume, and Kazuo Takimiya. 2016. "N,N′-Bis(2-cyclohexylethyl)naphtho[2,3-b:6,7-b′]dithiophene Diimides: Effects of Substituents" Molecules 21, no. 8: 981. https://doi.org/10.3390/molecules21080981






