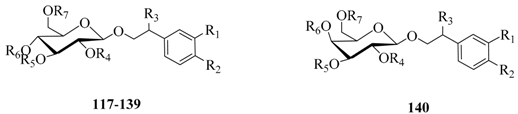Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics
Abstract
:1. Introduction
2. Phytochemistry
3. Pharmacological Activity
3.1. Neuroprotective Effects
3.2. Antioxidant Activity
3.3. Anti-Inflammatory Effect
3.4. Antibacterial and Antivirus Activity
3.5. Anti-Tumor Activity
3.6. Immunomodulatory Effect
3.7. Enzyme Inhibitory Activity
3.8. Other Pharmacological Effects
4. Pharmacokinetics
4.1. Pharmacokinetics of Echinacoside (117) and Acteoside (121)
4.2. Pharmacokinetics of Salidroside (123) and p-Tyrosol
4.3. Pharmacokinetics of Forsythoside (124)
4.4. Pharmacokinetics of Other PhGs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Georgiev, M.I.; Alipieva, K.; Orhan, I.; Abrashev, R.; Denev, P.; Angelova, M. Antioxidant and cholinesterases inhibitory activities of Verbascum xanthophoeniceum Griseb and its phenylethanoid glycosides. Food Chem. 2011, 128, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kirmizibekmez, H.; Ariburnu, E.; Masullo, M.; Festa, M.; Capasso, A.; Yesilada, E.; Piacente, S. Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 2012, 83, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Riguera, R. Phenylethanoid glycosides in plants: Structure and biological activity. Nat. Prod. Rep. 1994, 11, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.M.; Pang, H.H.; Wong, Y.H. Naturally occurring phenylethanoid glycosides: Potential leads for new therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef] [PubMed]
- Noiarsa, P.; Ruchirawat, S.; Kanchanapoom, T. Acanmontanoside, a new phenylethanoid diglycoside from Acanthus montanus. Molecules 2010, 15, 8967–8972. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.J.; Kindscher, K.; Timmermann, B.N. Cytotoxic cardiac glycosides and other compounds from Asclepias syriaca. J. Nat. Prod. 2012, 75, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nakamura, S.; Nakashima, S.; Oda, Y.; Matsumoto, T.; Fukaya, M.; Yano, M.; Yoshikawa, M.; Matsuda, H. Chemical structures of constituents from the whole plant of Bacopa monniera. J. Nat. Med. 2016, 70, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.F.; Yuan, J.Q.; Mei, Z.N.; Yang, X.Z. Phenolic glycosides from Boschniakia himalaica. Chin. Chem. Lett. 2012, 23, 579–582. [Google Scholar] [CrossRef]
- Bougandoura, A.; D’Abrosca, B.; Ameddah, S.; Scognamiglio, M.; Mekkiou, R.; Fiorentino, A.; Benayache, S.; Benayache, F. Chemical constituents and in vitro anti-infammatory activity of Cistanche violacea Desf (Orobanchaceae) extract. Fitoterapia 2016, 109, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.D.; Zeng, K.W.; Shi, S.P.; Zhao, M.B.; Jiang, Y.; Tu, P.F. Phenylethanoid glycosides with anti-inflammatory activities from the stems of Cistanche deserticola cultured in Tarim desert. Fitoterapia 2013, 89, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.N.; Morikawa, T.; Ninomiya, K.; Imura, K.; Yuan, D.; Yoshikawa, M.; Muraoka, O. Bioactive constituents from Chinese nature medicines: Four new acylated phenylethanoid oligoglycosides, kankanosides J1, J2, K1 and K2 from stems of Cistanche tubulosa. Chem. Pharm. Bull. 2010, 58, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Pan, Y.; Ninomiya, K.; Imura, K.; Matsuda, H.; Yoshikawa, M.; Yuan, D.; Muraoka, O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg. Med. Chem. 2010, 18, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Li, J.; Jiang, Y.; Zhao, M.B.; Tu, P.F. Chemical constituents from Cistanche sinensis (Orobanchaceae). Biochem. Syst. Ecol. 2013, 47, 21–24. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.J.; Li, P.F.; Yang, Y.B.; Wu, L.H.; Chou, G.X.; Wang, Z.T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and α-glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.G.; Guo, Y.M.; Luo, B.M.; Liu, W.M.; Wei, R.R.; Yang, C.X.; Ding, C.H.; Xu, X.F.; He, M.H. Hepatoprotective phenylethanoid glycosides from Cirsium setosum. Nat. Prod. Res. 2015, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.Z.; Zhai, Y.J.; Zhao, Z.X.; Zhang, C.X.; Lin, C.Z.; Zhu, C.C. Phenylethanoid glycosides from the stems of Callicarpa peii (hemostatic drug). Fitoterapia 2013, 84, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.L.; Jin, H.G.; Shin, J.E.; Hong, J.; Woo, E.R. Phenylethanoid glycosides from Digitals purpurea L. Bull. Korean Chem. Soc. 2011, 32, 1721–1724. [Google Scholar] [CrossRef]
- Li, C.; Dai, Y.; Zhang, S.X.; Duan, Y.H.; Liu, M.L.; Chen, L.Y.; Yao, X.S. Quinoid glycosides from Forsythia suspensa. Phytochemistry 2014, 104, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Xia, Y.G.; Yang, B.Y.; Liang, J.; Zhang, Q.B.; Li, G.Y. A new caffeoyl phenylethanoid glycosides from the unripe fruits of Forsythia suspense. Chin. J. Nat. Med. 2009, 7, 278–282. [Google Scholar] [CrossRef]
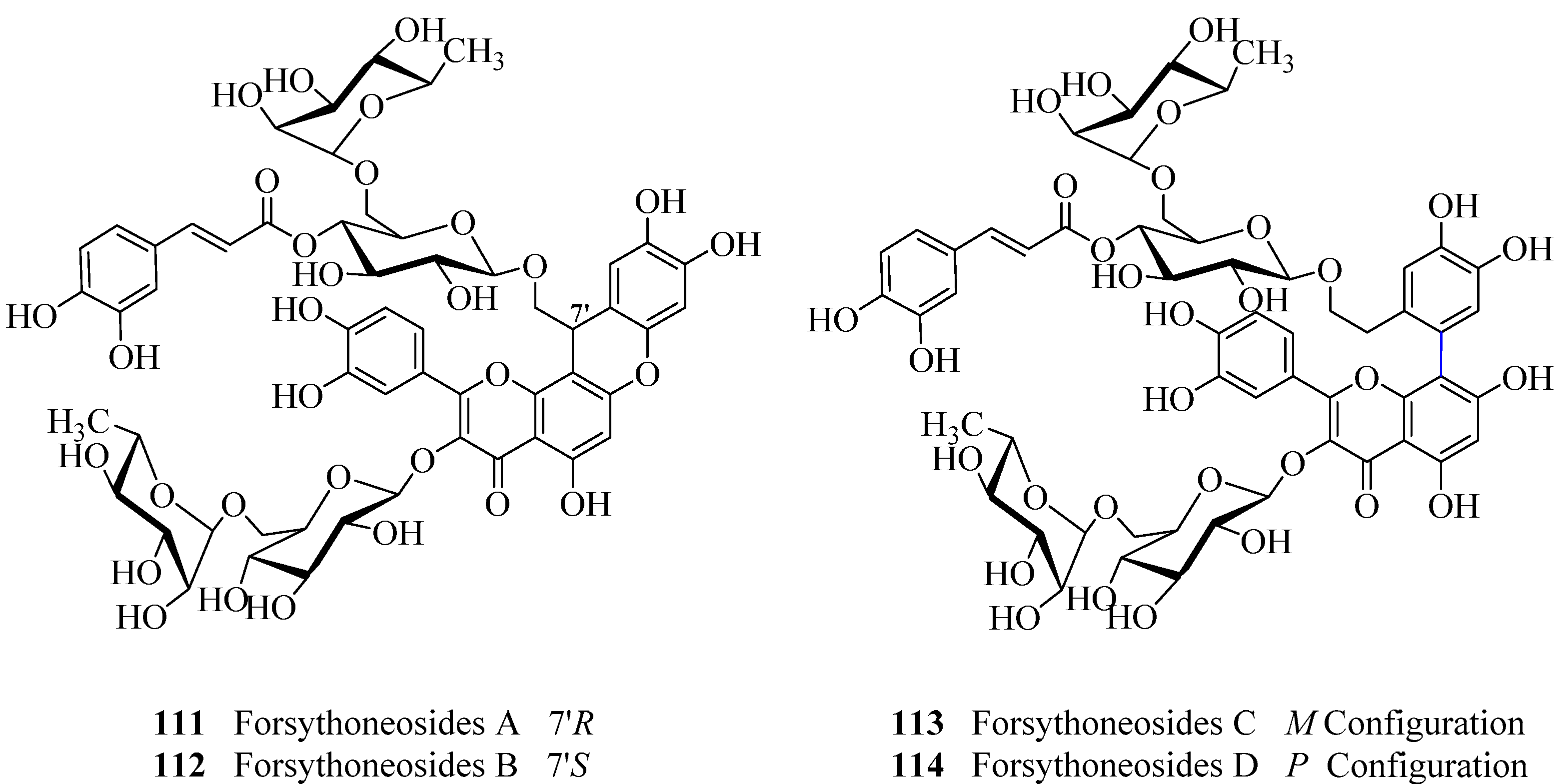
- Zhang, F.; Yang, Y.N.; Song, X.Y.; Shao, S.Y.; Feng, Z.M.; Jiang, J.S.; Li, L.; Chen, N.H.; Zhang, P.C. Forsythoneosides A-D, neuroprotective phenethanoid and flavone glycoside heterodimers from the fruits of Forsythia suspensa. J. Nat. Prod. 2015, 78, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.H.; Zhang, Y.M.; Chai, X.Y.; Sun, W.J. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa. Bioorg. Chem. 2012, 40, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.N.; Ma, Z.Q.; Liu, Y.; Guo, Y.Z.; Gu, Z.W. New phenylethanoid glycosides from the fruits of Forsythia suspense (Thunb.) Vahl. Molecules 2009, 14, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Zhang, H.G.; Li, X. Phenylethanoid glycosides from the bark of Fraxinus mandschurica. Chem. Nat. Compd. 2009, 3, 330–332. [Google Scholar] [CrossRef]
- Wu, H.F.; Zhu, Y.D.; Zhang, L.J.; Zou, Q.Y.; Chen, L.; Shen, T.; Wang, X.F.; Ma, G.X.; Hu, B.R.; Hu, W.C.; et al. A new phenylethanoid glycoside from Incarvillea compacta. J. Asian Nat. Prod. Res. 2015, 2, 1–7. [Google Scholar]
- Martin, F.; Hay, A.E.; Condoretty, V.R.Q.; Cressend, D.; Reist, M.; Gupta, M.P.; Carrupt, P.A.; Hostettmann, K. Antioxidant phenylethanoid glycosides and a neolignan from Jacaranda caucana. J. Nat. Prod. 2009, 72, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Julia, L.S.; Piccinelli, A.L.; Marzocco, S.; Leitão, S.G.; Lotti, C.; Autore, G.; Rastrelli, L. Phenylethanoid glycosides from Lantana fucata with in vitro anti-inflammatory activity. J. Nat. Prod. 2009, 72, 1424–1428. [Google Scholar]
- Sena Filho, J.G.; Nimmo, S.L.; Xavier, H.S.; Barbosa-Filho, J.M.; Cichewicz, R.H. Phenylethanoid and lignan glycosides from polar extracts of Lantana, a genus of verbenaceous plants widely used in traditional herbal therapies. J. Nat. Prod. 2009, 72, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Chen, Z.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012, 348, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kondratyuk, T.P.; Jermihov, K.C.; Marler, L.E.; Qiu, X.; Choi, Y.; Cao, H.M.; Yu, R.; Sturdy, M.; Huang, R.; et al. Bioactive compounds from the fern Lepisorus contortus. J. Nat. Prod. 2011, 74, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Deng, J.; Wang, Y.H. A new phenylethanoid glucoside from Lagotis brevituba. Chin. J. Chin. Mater. Med. 2009, 16, 2054–2056. [Google Scholar]
- Porter, E.A.; Kite, G.C.; Veitch, N.C.; Geoghegan, I.A.; Larsson, S.; Simmonds, M.S.J. Phenylethanoid glycosides in tepals of Magnolia salicifolia and their occurrence in flowers of Magnoliaceae. Phytochemistry 2015, 117, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.Z.; Yan, R.Y.; Yang, B. Phenylethanoid glycosides and phenolic glycosides from stem bark of Magnolia officinalis. Phytochemistry 2016, 127, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Yan, R.Y.; Liang, R.X.; Wang, W.H.; Yang, B. Bioactive polar compounds from stem bark of Magnolia officinalis. Fitoterapia 2012, 83, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, M.F.; Liu, Y.L.; Xu, Q.M.; Yang, S.L. Phenylethanoid glycosides from Monochasma savatieri and their anticomplement activity through the classical pathway. Planta Med. 2012, 78, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Miyase, T.; Yoshizaki, F. New phenolic compounds from Meehania urticifolia. J. Nat. Med. 2011, 65, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Kuramoto, H.; Kamei, I.; Yoshikawa, M.; Muraoka, O. Phenylethanoid and phenylpropanoid glycosides with melanogenesis inhibitory activity from the flowers of Narcissus tazetta var chinensis. J. Nat. Med. 2015, 70, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.X.; Duan, W.L.; Liu, P.; Yang, Y.L.; Yin, W.P. Secondary metabolites from the roots of Phlomis umbrosa. J. Asian Nat. Prod. Res. 2011, 13, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Deng, R.X.; Duan, H.Q.; Yin, W.P.; Zhao, T.Z. Phenylethanoid glycosides from the roots of Phlomis umbrosa. J. Asian Nat. Prod. Res. 2009, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, B.; Riaz, N.; Saleem, M.; Naveed, M.A.; Ashraf, M.; Alam, U.; Rafiq, H.M.; Tareen, R.B.; Jabbar, A. Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity. Phytochemistry 2013, 96, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Sun, Y.C.; Chen, G. A new phenylethanoid glucoside from Plantago depressa Willd. Nat. Prod. Res. 2013, 27, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Yoshimura, A.; Yoshimura, M.; Yoshida, T. Isolation and characterization of phenolic antioxidants from Plantago Herb. Molecules 2012, 17, 5459–5466. [Google Scholar] [CrossRef] [PubMed]
- Phakeovilay, C.; Disadee, W.; Sahakitpichan, P.; Sitthimonchai, S.; Kittakoop, P.; Ruchirawat, S.; Kanchanapoom, T. Phenylethanoid and flavone glycosides from Ruellia tuberosa L. J. Nat. Med. 2013, 67, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Delazara, A.; Delnavaziab, M.R.; Naharc, L.; Moghadama, S.B.; Mojarabab, M.; Guptad, A.; Williamsd, A.S.; Rahmane, M.M.; Sarkerf, S.D. Lavandulifolioside B: A new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat. Prod. Res. 2011, 25, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.D.; Festa, C.; Zollo, F.; Incollingo, F.; Raimo, G.; Evangelista, G.; Iorizzi, M. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chem. 2012, 133, 21–28. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Pascarella, M.T.; Letizia, M.; Uzzo, P.; Piscopo, V.; Fiorentino, A. Antioxidant efficacy of iridoid and phenylethanoid glycosides from the medicinal plant Teucrium chamaedris in cell-free systems. Bioorgan. Med. Chem. 2009, 17, 6173–6179. [Google Scholar] [CrossRef] [PubMed]
- Taskova, R.M.; Kokubun, T.; Ryan, Y.K.G.; Garnock-jones, P.J.; Jensen, S.R. Phenylethanoid and iridoid glycosides in the New Zealand snow hebes (Veronica, Plantaginaceae). Chem. Pharm. Bull. 2010, 58, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Taskova, R.M.; Kokubun, T.; Ryan, K.G.; Garnock-Jones, P.J.; Jensen, S.R. Iridoid and phenylethanoid glucosides from Veronica lavaudiana. J. Nat. Prod. 2011, 74, 1477–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taskova, R.M.; Kokubun, T.; Garnock-Jones, P.J.; Jensen, S.R. Iridoid and phenylethanoid glycosides in the New Zealand sun hebes (Veronica; Plantaginaceae). Phytochemistry 2012, 77, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H. Lianqiaoxinoside B, a novel caffeoyl phenylethanoid glycoside from Forsythia suspensa. Molecules 2011, 16, 5674–5681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Wen, Q.W.; Lin, X.; Zhang, S.J.; Li, Y.X.; Guo, Y.J.; Huang, B. A new phenylethanoid glycoside with antioxidant and anti-HBV activity from Tarphochlamys affinis. Arch. Pharm. Res. 2014, 37, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Bhangalia, S.; Singh, H.P. A new phenylethanoid glucoside from Jacaranda mimosifolia. Nat. Prod. Res. 2013, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, Y.; Hao, X.J.; Yang, F.M.; Sun, Q.Y.; Morris-Natschke, S.L.; Lee, K.H.; Wang, Y.H.; Long, C.L. Indole alkaloid glycosides from the aerial parts of Strobilanthes cusia. J. Nat. Prod. 2014, 77, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H.; Ellgring, H. Parkinson′s disease-medical education and psychosocial aspects. Patient Educ. Couns. 1995, 26, 71–79. [Google Scholar] [CrossRef]
- Seniuk, N.A.; Tatton, W.G.; Greenwood, C.E. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990, 527, 7–20. [Google Scholar] [CrossRef]
- Hantraye, P.; Varastet, M.; Peschanski, M.; Riche, D.; Cesaro, P.; Willer, J.C.; Maziere, M. Stable Parkinsonian syndrome and uneven loss of striatal dopamine fibres following chronic MPTP administration in baboons. Neuroscience 1993, 53, 169–178. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Hess, A.; Duvoisin, R.C. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 1984, 224, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Trevor, A.; Castagnoli, N., Jr. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 1984, 120, 574–578. [Google Scholar] [CrossRef]
- Geng, X.C.; Tian, X.F.; Tu, P.F.; Pu, X.P. Neuroprotective effects of echinacoside in the mouse MPTP model of Parkinson’s disease. Eur. J. Pharmacol. 2007, 564, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Lu, J.H.; Li, Q.; Zhao, Y.Y.; Pu, X.P. Pedicularioside A from Buddleia lindleyana inhibits cell death induced by 1-methyl-4-phenylpyridinium ions (MPP+) in primary cultures of rat mesencephalic neurons. Eur. J. Pharmacol. 2008, 579, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Reddy, P.H. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s disease. J. Neurochem. 2006, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.; Davis, J.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Wang, H.Q.; Xu, Y.X.; Zhu, C.Q. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox. Res. 2012, 21, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Tsai, H.J.; Hung, T.H.; Chen, C.C.; Lee, C.Y.; Wu, C.H.; Wang, P.Y.; Liao, N.C. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS ONE 2012, 7, e45763. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Xu, Y.X.; Yan, J.; Zhao, X.Y.; Sun, X.B.; Zhang, Y.P.; Guo, J.C.; Zhu, C.Q. Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Res. 2009, 1283, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.M.; Gao, L.; Huo, S.X.; Liu, X.M.; Yan, M. The mechanism of memory enhancement of acteoside (verbascoside) in the senescent mouse model induced by a combination of gal and AlCl3. Phytother. Res. 2015, 29, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, E.J.; Lee, H.S.; Kim, Y.C. Acteoside of Callicarpa dichotoma attenuates scopolamine-induced memory impairments. Biol. Pharm. Bull. 2006, 29, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Howard, B.; Yatin, S.; Koppal, T.; Drake, J.; Hensley, K.; Aksenov, M.; Aksenova, M.; Subramaniam, R.; Varadarajan, S.; et al. Elevated oxidative stress in models of normal brain aging and Alzheimer’s disease. Life Sci. 1999, 65, 1883–1892. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, J.Y.; Tu, P.F.; Jiang, Y.; Li, Z.B.; Wang, Y.H. Echinacoside rescues the SHSY5Y neuronal cells from TNF alpha-induced apoptosis. Eur. J. Pharmacol. 2004, 505, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kuang, R.; Sun, Y.G.; Yuan, W.; Lei, L.; Zheng, X.X. Protective effects of echinacoside, one of the phenylethanoid glycosides, on H2O2-induced cytotoxicity in PC12 cells. Planta Med. 2009, 75, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Si, C.L.; Shen, T.; Jiang, Y.Y.; Wu, L.; Yu, G.J.; Ren, X.D.; Xu, G.H.; Hu, W.C. Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem. Toxicol. 2013, 59, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Zhu, P.; Huang, L.; Ye, J. The research progress of Callicarpa kwangtungensis Chun. Mod. Chin. Med. 2011, 13, 37–39. [Google Scholar]
- Harput, U.S.; Genc, Y.; Saracoglu, I. Cytotoxic and antioxidative activities of Plantago lagopus L. and characterization of its bioactive compounds. Food Chem. Toxicol. 2012, 50, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xie, Z.Y.; Liu, G.H.; Sun, X.M.; Peng, G.T.; Lin, B.Q.; Liao, Q.F. Isolation, identification and activities of natural antioxidants from Callicarpa kwangtungensis Chun. PLoS ONE 2014, 9, e93000. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Hu, J.P.; Rena, K.; Du, N.S. Structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity. Zhong Yao Cai 2009, 32, 1067–1069. [Google Scholar] [PubMed]
- Lodise, T.P., Jr.; Patel, N.; Kwa, A.; Graves, J.; Furuno, J.P.; Graffunder, E.; Lomaestro, B.; McGregor, J.C. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa blood stream infection: Impact of delayed appropriate antibiotic selection. Antimicrob. Agents Chem. 2007, 51, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.G.; Snelling, A.M. Pseudomonas aeruginosa: A formidable and ever-present adversary. J. Hosp. Infect. 2009, 73, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; He, W.J.; Mo, L.; Shi, M.F.; Zhu, Y.Y.; Pan, S.; Li, X.R.; Xu, Q.M.; Yang, S.L. Antimicrobial, anti-inflammatory activities and toxicology of phenylethanoid glycosides from Monochasma savatieri Franch Ex Maxim. J. Ethnopharmacol. 2013, 149, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.S.; Oh, B.K.; Pak, J.H.; Yim, S.H.; Gurunathan, S.; Kim, Y.P.; Lee, K.J. Acteoside improves survival in cecal ligation and puncture-induced septic mice via blocking of high mobility group box 1 release. Mol. Cells 2013, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, Z.; Kahraman, C.; Tatlı, II.; Akkol, E.K.; Süntar, I.; Keles, H. Bioassay-guided isolation of anti-inflammatory, antinociceptive and wound healer glycosides from the flowers of Verbascum mucronatum Lam. J. Ethnopharmacol. 2011, 136, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, N.; Delporte, C.; Apablaza, C.; Farías, M.; Goïty, L.; Arrau, S.; Negrete, R.; Castro, C.; Miranda, H. Antinociceptive activity of Buddleja globose (matico) in several models of pain. J. Ethnopharmacol. 2008, 119, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Okada, Y.; Kupeli Akkol, E.; Duman, H.; Okuyama, T.; Calis, I. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- Penido, C.; Costa, K.A.; Futuro, D.O.; Paiva, S.R.; Kaplan, M.A.C.; Figueiredo, M.R.; Henriques, M.G.M.O. Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis. J. Ethnopharmacol. 2006, 104, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Obermeier, F.; Paper, D.H.; Balan, K.; Dunger, N.; Menzel, K.; Falk, W.; Schoelmerich, J.; Herfarth, H.; Rogler, G. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2007, 148, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Song, T.Y.; Liang, Y.C.; Hu, M.L. Acteoside and 6-O-acetylacteoside downregulate cell adhesion molecules induced by IL-1β through inhibition of ERK and JNK in human vascular endothelial cells. J. Agric. Food Chem. 2009, 57, 8852–8859. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.Y.; Luo, F.; Jiang, W.J.; Zhu, L.P.; Gao, J.; He, H.; Wei, T.T.; Gong, S.L.; Yan, T.H. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-κB pathway in vivo and in vitro. Int. Immunopharmac. 2015, 28, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.P.; Shao, M.M.; Song, X.; Wu, X.L.; Qi, L.; Zheng, K.; Fan, L.; Liao, C.H.; Li, C.Y.; He, J.; et al. Anti-influenza virus effects of crude phenylethanoid glycosides isolated from Ligustrum purpurascens via inducing endogenous interferon-γ. J. Ethnopharmacol. 2015, 179, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Nazemiyeh, H.; Rahman, M.M.; Gibbons, S.; Nahar, L.; Delazar, A.; Ghahramani, M.A.; Talebpour, A.H.; Sarker, S.D. Assessment of the antibacterial activity of phenylethanoid glycosides from Phlomis lanceolate against multiple-drug-resistant strains of Staphylococcus aureus. J. Nat. Med. 2008, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhao, Y.; Norman, V.L.; Starks, C.M.; Rice, S.M.; Goering, M.G.; O′Neil-Johnson, M.; Eldridge, G.R.; Hu, J.F. Antibiofilm phenylethanoid glycosides from Penstemon centranthifolius. Phytother. Res. 2010, 24, 778–781. [Google Scholar] [PubMed]
- Prusky, D.; Keen, N.T. Involvement of preformed antifungal compounds in the resistance of subtropical fruits to fungal decay. Plant. Dis. 1993, 77, 114–119. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, A.P.; Fan, Z.M.; Du, Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol. Rep. 2015, 33, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Kim, H.G.; Choi, J.H.; Park, B.H.; Jeong, M.H.; Jeong, T.C.; Jeong, H.G. ACT inhibits PMA-induced matrix metalloproteinase-9 expression via CaMK/ERK- and JNK/NF-ƙB-dependent signaling. Mol. Nutr. Food Res. 2011, 55, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, A.; Samara, P.; Tsitsilonis, O.; Skaltsa, H. Polar constituents of Marrubium thessalum Boiss. & Heldr. (Lamiaceae) and their cytotoxic/cytostatic activity. Phytother. Res. 2012, 26, 1800–1806. [Google Scholar] [PubMed]
- Wolf, D.; Hallmann, R.; Sass, G.; Sixt, M.; Küsters, S.; Fregien, B.; Trautwein, C.; Tiegs, G. TNF-𝛼-induced expression of adhesion molecules in the liver is under the control of TNFR1-relevance for concanavalin A-induced hepatitis. J. Immunol. 2001, 166, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.M.; Yu, X.F.; Guo, C.Q.; Wang, H.X.; Qian, J.; Yi, H.F.; Lu, X.L.; Lv, Z.P.; Subjeck, J.R.; Zhou, H.P.; et al. Scavenger receptor A restrains T-cell activation and protects against concanavalin A-induced hepatic injury. Hepatology 2013, 57, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.J.; Zou, Y.; Liu, S.S.; Wang, J.; Zhu, J.L.; Li, J.B.; Bo, L.L.; Deng, X.M. Salidroside attenuates concanavalin A-induced hepatitis via modulating cytokines secretion and lymphocyte migration in mice. Mediat. Inflamm. 2014, 2014, 314081. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, C.Y.; Zeng, Y.; Wu, H.Q.; Huang, Z.; Zhang, J.; Hong, R.S.; Chen, X.X.; Wang, L.Y.; Hu, X.P.; et al. Immunomodulatory effects of crude phenylethanoid glycosides from Ligustrum purpurascens. J. Ethnopharmacol. 2012, 144, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.F.; Tang, Y.F.; Nie, S.P.; Wan, Y.; Xie, M.Y.; Xie, X.M. Effect of phenylethanoid glycosides and polysaccharides from the seed of Plantago asiatica L. on the maturation of murine bone marrow-derived dendritic cells. Eur. J. Pharmacol. 2009, 620, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Prescott, T.A.K.; Veitch, N.C.; Simmonds, M.S.J. Direct inhibition of calcineurin by caffeoyl phenylethanoid glycosides from Teucrium chamaedrys and Nepeta cataria. J. Ethnopharmacol. 2011, 137, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Alipieva, K.; Orhan, I.E. Cholinesterases inhibitory and antioxidant activities of Harpagophytum procumbens from in vitro systems. Phytother. Res. 2012, 26, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, Y.S.; Kim, S.H.; Bae, Y.S.; Lim, S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011, 34, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Imamura, M.; Akaki, J.; Fujikura, S.; Pan, Y.; Yuan, D.; Yoshikawa, M.; Jia, X.G.; Li, Z.; et al. Acylated phenylethanoid glycosides, echinacoside and acteoside from Cistanche tubulosa, improve glucose tolerance in mice. J. Nat. Med. 2014, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Tang, H.; Xiao, F.R.; Gong, J.L.; Peng, Y.; Meng, X.L. Protective effect of salidroside from Rhodiolae. Radix on diabetes-induced oxidative stress in mice. Molecules 2011, 16, 9912–9924. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, M.; Miyamae, Y.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Inhibition of amyloid β aggregation by acteoside, a phenylethanoid glycoside. Biosci. Biotechnol. Biochem. 2013, 77, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Marcoccia, D.; Georgiev, M.I.; Alipieva, K.I.; Lorenzetti, S. Inhibition of the DHT-induced PSA secretion by Verbascum xanthophoeniceum and Serenoa repens extracts in human LNCaP prostate epithelial cells. J. Ethnopharmacol. 2014, 155, 616–625. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Fang, T.H.; Ma, X.; Zhang, K.; Ma, Z.Z.; Tu, P.F. Echinacoside elicits endothelium-dependent relaxation in rat aortic rings via an NO-cGMP pathway. Planta Med. 2009, 75, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zong, C.J.; Liu, F.; Fang, L.; Cai, R.L.; Shi, Y.; Chen, X.; Qi, Y. Evaluation of the intestinal transport of a phenylethanoid glycoside-rich extract from Cistanche deserticola across the Caco-2 cell monolayer model. PLoS ONE 2015, 10, e0116490. [Google Scholar] [CrossRef] [PubMed]
- Matthias, A.; Blanchfield, J.T.; Penman, K.G.; Toch, I.; Lang, C.S.; de Voss, J.J.; Lehmann, R.P.; Clin, J. Permeability studies of alkylamides and caffeic acid conjugates from echinacea using a Caco-2 cell monolayer model. J. Clin. Pharm. Ther. 2004, 29, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Yang, X.L.; Yang, Z.L.; Kou, J.P.; Li, F. Enhancement of absorption and bioavailability of echinacoside by verapamil or clove oil. Drug Des. Dev. Ther. 2015, 9, 4685–4693. [Google Scholar]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2010, 2, 1–12. [Google Scholar] [CrossRef]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhut, K.; Hogger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In vitro antioxidant activity of coffee compounds and their metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar]
- Koo, K.A.; Kim, S.H.; Oh, T.H.; Kim, Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006, 79, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Muller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D′Arcy, B.R.; Williams, B.A. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Q.; Shi, H.M.; Jin, W.; Zhang, K.; Jiang, Y.; Zhao, M.B.; Tu, P.F. Metabolism of echinacoside, a good antioxidant, in rats: Isolation and identification of its biliary metabolites. Drug Metab. Dispos. 2009, 37, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hao, H.P.; Wang, G.J.; Tu, P.F.; Jiang, Y.; Liang, Y.; Dai, L.; Yang, H.; Lai, L.; Zheng, C.N.; et al. An approach to identifying sequential metabolites of a typical phenylethanoid glycoside, echinacoside, based on liquid chromatography-ion trap-time of flight mass spectrometry analysis. Talanta 2009, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Song, Z.H.; Tu, P.F.; Li, Y.Z.; Wu, L.J.; Chen, F.K. Separation of echinacoside by reversed-phase preparative high performance liquid chromatography. Chin. J. Chromatogr. 2001, 19, 200–202. [Google Scholar]
- Li, Y.; Zhou, G.S.; Xing, S.H.; Tu, P.F.; Li, X.B. Identification of echinacoside metabolites produced by human intestinal bacteria using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 6764–6771. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xiong, A.Z.; Li, P.F.; Yang, Q.M.; Yang, Li.; Wang, Z.T. Identification of acteoside and its major metabolites in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B 2013, 940, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Pan, Y.N.; Xu, X.T.; Zhang, W.J.; Wu, X.; Qu, S.H.; Liu, X.Q. The metabolic profile of acteoside produced by human or rat intestinal bacteria or intestinal enzyme in vitro employed UPLC-Q-TOF-MS. Fitoterapia 2016, 109, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.S.; Peng, Y.; Tu, P.F.; Li, X.B. Screening and identification of three typical phenylethanoid glycosides metabolites from Cistanches Herba by human intestinal bacteria using UPLC/Q-TOF-MS. J. Pharm. Biomed. 2016, 118, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Huo, S.X.; Gao, L.; Li, J.M.; Lin, J.; Cai, L.M.; Yan, M.; Huang, Y.; KAISAIER, A. Pharmacokinetic study on acetoside in rats. Chin. J. Chin. Mater. Med. 2012, 37, 417–420. [Google Scholar]
- Gan, P.; Huo, S.X.; Bai, P.; Gao, L.; Peng, X.M.; He, Y.; Yan, M. Pharmacokinetics and tissue distribution of acteoside in rats. Chin. Pharmacol. Bull. 2014, 30, 417–420. [Google Scholar]
- Li, Y.J.; Gan, L.; Li, G.Q.; Deng, L.; Zhang, X.S.; Deng, Y.L. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J. Pharm. Biomed. 2014, 89, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Lin, L.C.; Sung, J.S.; Tsai, T.H. Determination of acetoside in Cistanche deserticola and Boschniakia rossica and its pharmacokinetics in freely-moving rats using LC-MS/MS. J. Chromatogr. B 2006, 844, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Q.; Shi, H.M.; Wu, X.M.; Li, Y.Z.; Chen, J.J.; Tu, P.F. Determination of echinacoside in rat serum by reversed-phase high-performance liquid chromatography with ultraviolet detection and its application to pharmacokinetics and bioavailability. J. Chromatogr. B 2006, 844, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, G.J.; Hao, H.P.; Tu, P.F.; Jiang, Y.; Wang, Q.; Zhang, Y.; Zheng, C.N.; Wang, Y.X.; Dai, L. A sensitive and specific liquid chromatography/tandem mass spectrometry method for determination of echinacoside and its pharmacokinetic application in rats. Biomed. Chromatogr. 2009, 23, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Q.; Sun, J.B.; Wang, F.Q.; Zeng, P. Simultaneous separation and determination of four phenylethanoid glycosides in rat plasma sample after oral administration of Cistanche salsa extract by microemulsion liquid chromatography. J. Chromatogr. B 2014, 951–952, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Q.; Sun, J.B.; Sun, X.L.; Ping, Z. Two-phase hollow fiber liquid phase microextraction based on magnetofluid for simultaneous determination of echinacoside, tubuloside B, acteoside and isoacteoside in rat plasma after oral administration of Cistanche salsa extract by high performance liquid chromatography. J. Pharm. Biomed. 2014, 94, 30–35. [Google Scholar]
- Guo, N.; Hu, Z.W.; Fan, X.X.; Zheng, J.; Zhang, D.H.; Xu, T.; Yu, T.; Wang, Y.; Li, H.Y. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules 2012, 17, 4733–4754. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.W.; Wang, Z.M.; Liu, Y.; Wu, Y.; Han, X.J.; Zheng, J.; Yan, X.F.; Wang, Y. Metabolite profile of salidroside in rats by ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry and high-performance liquid chromatography coupled with quadrupole-linear ion trap mass spectrometry. J. Agric. Food Chem. 2015, 63, 8999–9005. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ding, W.M.; Wang, Y.; Hu, Z.W.; Wang, Z.M.; Wang, Y. An LC–MS/MS method for the determination of salidroside and its metabolite p-tyrosol in rat liver tissues. Pharm. Biol. 2014, 52, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Bartholome, R.; Haenen, G.; Hollman, P.C.H.; Bast, A.; Dagnelie, P.C.; Roos, D.; Keijer, J.; Kroon, P.A.; Needs, P.W.; Arts, I.C.W. Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by β-glucuronidase from neutrophils. Drug Metab. Pharm. 2010, 25, 379–387. [Google Scholar] [CrossRef]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.R.; Zhang, X.D.; Lu, G.C. Development of an HPLC method for the determination of salidroside in beagle dog plasma after administration of salidroside injection: Application to a pharmacokinetics study. J. Sep. Sci. 2007, 30, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, L.; Wen, T.; Liu, Y.C.; Wang, D.L.; He, Y.X.; Liang, Y.; Liu, X.D.; Xie, L.; Wang, G.J.; Wei, W.Z. Development and validation of a liquid chromatographic/electrospray ionization mass spectrometric method for the determination of salidroside in rat plasma: Application to the pharmacokinetics study. J. Chromatogr. B 2008, 861, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Yao, H.T.; Hsieh, S.H.; Lu, T.J.; Yeh, T.K. Quantitative determination of salidroside in rat plasma by on-line solid-phase extraction integrated with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2007, 857, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.N.; Pan, R.L.; Liao, Y.H.; Chen, Y.; Tang, J.T.; Chang, Q. An LC-MS/MS method for determination of forsythiaside in rat plasma and application to a pharmacokinetic study. J. Chromatogr. B 2010, 878, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Di, L.Q.; Wang, J.; Shan, J.J.; Liu, S.J.; Ju, W.Z.; Cai, B.C. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharm. Sin. 2012, 33, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhang, R.Q.; Peng, C. Assessment and modulation of forsythiaside absorption with MDCKII cells and validation with in situ intestinal experiment. Eur. J. Drug Metab. Pharm. 2012, 37, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qin, K.M.; Shan, J.J.; Ju, W.Z.; Liu, S.J.; Cai, B.C.; Di, L.Q. Improvement of intestinal absorption of forsythoside A in weeping forsythia extract by various absorption enhancers based on tight junctions. Phytomedicine 2012, 20, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.H.; Peng, Y.; Wang, M.Y.; Chen, D.F.; Li, X.B. In vitro human fecal microbial metabolism of forsythoside A and biological activities of its metabolites. Fitoterapia 2014, 99, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xuan, Z.; Ma, Y.; Liu, Y.; Lu, H.; Sun, T. Pharmacokinetics of forsythoside after intravenous administration in beagle dogs. Eur. J. Drug Metab. Pharm. 2009, 34, 101–105. [Google Scholar] [CrossRef]
- Li, Y.X.; Jiang, X.H.; Liang, H.Y.; Li, X. Determination of forsythiaside in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies. Biomed. Chromatogr. 2008, 22, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, X.Y.; Guo, J.H.; Li, W.; Ma, X.H.; Zhu, Y.H. Pharmacokinetic study of unbound forsythiaside in rat blood and bile by microdialysis coupled with HPLC method. Eur. J. Drug Metab. Pharm. 2012, 37, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.X.; Wei, W.; Quan, L.H.; Liu, C.Y.; Chang, Q.; Liao, Y.H. An LC–MS/MS method for the simultaneous determination of chlorogenic acid, forsythiaside A and baicalin in rat plasma and its application to pharmacokinetic study of Shuang-huang-lian in rats. J. Pharm. Biomed. 2010, 52, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Wang, Y.; Deng, Y.T.; Shi, T.R.; Liu, X.Y.; Sun, X.L.; Li, X.Y.; Zhou, D. A rapid determination of drug candidate tyrosol galactoside in rat plasma by HPLC and its application to the pharmacokinetics study. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 131–136. [Google Scholar] [CrossRef] [PubMed]


| No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Source | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acanmontanoside | OH | OH | H | H | 4-O-Syringoyl-Rha | Caffeoyl | H | Acanthus montanus | - a | [5] |
| 2 | Kansanoside A | H | H | H | Gal | H | H | Xyl | Asclepias syriaca | - a | [6] |
| 3 | Bacomoside A | OH | OH | =O | p-hydroxy-benzoyl | H | H | H | Bacopa monniera | - b | [7] |
| 4 | Bacomoside B1/B2 | OH | OH | OCH3 | Caffeoyl | H | H | H | B. monniera | Inhibitory effects on Aβ42 aggregation | [7] |
| 5 | Himaloside A | OCH3 | OH | H | Acetyl | Glc(1→4)Rha | Caffeoyl | H | Boschniakia himalaica | Antibacterial activity | [8] |
| 6 | Himaloside B | OH | OH | H | H | H | H | cis-Caffeoyl | B. himalaica | Antibacterial activity | [8] |
| 7 | Z-Tubuloside D | OH | OH | H | Acetyl | 2,3,4-tri-O-Acetyl-Rha | Coumaroyl | Glc | Cistanche violacea | - a | [9] |
| 8 | Cistanoside J | OCH3 | OH | H | Acetyl | Rha | H | Feruloyl | C. deserticola | Anti-inflammatory activity | [10] |
| 9 | Cistanoside K | OCH3 | OH | H | Acetyl | Rha | H | Caffeoyl | C. deserticola | Anti-inflammatory activity | [10] |
| 10 | Cistanoside L | OCH3 | OCH3 | H | H | Rha | H | Feruloyl | C. deserticola | - b | [10] |
| 11 | Cistanoside M | OCH3 | OH | H | H | Rha | H | Coumaroyl | C. deserticola | Anti-inflammatory activity | [10] |
| 12 | Cistanoside N | OCH3 | OH | H | Acetyl | Rha | H | 3-O-Glc-Caffeoyl | C. deserticola | Anti-inflammatory activity | [10] |
| 13 | Kankanoside J1/J2 | OH | OH | OCH3 | Acetyl | Rha | Caffeoyl | H | C. tubulosa | - a | [11] |
| 14 | Kankanoside K1/K2 | OH | OH | OCH3 | H | Rha | Caffeoyl | Glc | C. tubulosa | Hepatoprotective activity | [11] |
| 15 | Kankanoside H1/H2 | OH | OH | H | Acetyl | Rha | trans/cis-Coumaroyl | Glc | C. tubulosa | - a | [12] |
| 16 | Kankanoside I | H | H | H | H | Rha | Caffeoyl | Glc | C. tubulosa | - a | [12] |
| 17 | Cistansinenside B | OH | OCH3 | H | Acetyl | Rha | Caffeoyl | Rha | C. sinensis | - a | [13] |
| 18 | Bunginoside A | H | OH | H | 5-O-glycosmisyl-Api | H | H | H | Clerodendrum bungei | - a | [14] |
| 19 | 3″,4″-di-O-acetylmartynoside | OH | OCH3 | H | H | 3,4-di-O-Acetyl-Rha | Feruloyl | H | C. bungei | - b | [14] |
| 20 | β-d-Glucopyranoside,1″-O-(7S)-7-(3-methoxyl-4-hydroxy-phenyl)-7-methoxyethyl-3″-α-l-rhamn-opyranosyl-4″-[(8E)-7-(3-metho-xyl-4-hydroxy-phenyl)-8-propenoate] | OCH3 | OH | OCH3 | H | Rha | Feruloyl | H | Cirsium setosum | - b | [15] |
| 21 | β-d-Glucopyranoside,1″-O-(7S)-7-(3-methoxyl-4-hydroxy-phenyl)-7-methoxyethyl-3″-α-l-rhamn-opyranosyl-4″-[(8E)-7-(4-hydrox-yphenyl)-8-propenoate] | OCH3 | OH | OCH3 | H | Rha | Coumaroyl | H | C. setosum | Hepatoprotective effect | [15] |
| 22 | Peiioside B | OH | OH | H | H | Rha | H | Api | Callicarpa peii | -a | [16] |
| 23 | Purpureaside D | OH | OH | H | H | H | Feruloyl | Rha | Digitalis purpurea | Antioxidant activity | [17] |
| 24 | Purpureaside E | OH | OH | H | H | Glc | Feruloyl | Rha | D. purpurea | Antioxidant activity | [17] |
| 25 | Forsythenside K | OH | OH | H | H | H | Coumaroyl | Rha | Forsythia suspensa | Antiviral activity | [18] |
| 26 | Lianqiaoxinside A | OH | OH | H | H | Caffeoyl | H | Rha | F. suspensa | Antibacterial activity | [19] |
| 27 | 2-(3,4-Dihydroxyphenyl)-2-oxo-ethyl-O-α-l-hamnopyranosyl-(1→6)-(4-O-caffeoyl)-β-d-glucopyranoside | OH | OH | =O | H | H | Caffeoyl | Rha | F. suspensa | - b | [20] |
| 28 | Forsythoside A 4′-O-β-d-glucopyranoside | OH | OH | H | H | H | 4-O-Glc-Caffeoyl | Rha | F. suspensa | - b | [20] |
| 29 | Isoforsythoside | OH | OH | H | H | Caffeoyl | H | Rha | F. suspensa | Antioxidant and antibacterial effects | [21] |
| 30 | Forsythoside H | OH | OH | H | Caffeoyl | H | H | Rha | F. suspensa | - a | [22] |
| 31 | Forsythoside I | OH | OH | H | H | Caffeoyl | H | Rha | F. suspensa | - a | [22] |
| 32 | Forsythoside J | OH | OH | H | Caffeoyl | H | H | Xyl | F. suspensa | - a | [22] |
| 33 | Calceolarioside A-2′-α-l-rhamnopyranoside | OH | OH | H | Rha | H | Caffeoyl | H | Fraxinus mandschurica | - a | [23] |
| 34 | 3′′′-O-Methylcampneoside I | OH | OH | OCH3 | H | Rha | Feruloyl | H | Incarvillea compacta | Hepatoprotective and antioxidant effects | [24] |
| 35 | 6′-O-(cis-1,4-Dihydroxycyclohex-nacetyl) acteoside | OH | OH | H | H | Rha | Caffeoyl | cis-1,4-Dihydroxy-cyclohexanacetyl | Jacaranda caucana | Antioxidant capacity | [25] |
| 36 | 6′-O-(1-Hydroxy-4-oxo-cyclohexanacetyl) acteoside | OH | OH | H | H | Rha | Caffeoyl | 1-Hydroxy-4-oxo-cyclohexanacetyl | J. caucana | Antioxidant capacity | [25] |
| 37 | Fucatoside A | OH | OH | H | Api | H | Caffeoyl | H | Lantana fucata | - b | [26] |
| 38 | Fucatoside B | OH | OH | H | Xyl | Api | Caffeoyl | H | L. fucata | - b | [26] |
| 39 | Fucatoside C | OH | OH | H | Api | Api | Caffeoyl | H | L. fucata | Anti-inflammatory effect | [26] |
| 40 | Raduloside | OH | OH | H | H | Api | Caffeoyl | Api(1→4)Xyl | L. radula | - b | [27] |
| 41 | Leonoside E | OCH3 | OH | H | H | Ara(1→2)Rha | H | H | Leonurus japonicus | Hepatoprotective activity | [28] |
| 42 | Leonoside F | OCH3 | OH | H | H | Rha | H | Glc | L. japonicus | Hepatoprotective activity | [28] |
| 43 | β-(4-Hydroxyphenyl) ethyl-4-O-E-caffeoyl-O-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | H | OH | H | Api | H | Caffeoyl | H | Lepisorus contortus | Cytotoxity | [29] |
| 44 | β-(3,4-Dihydroxyphenyl) ethyl-6-O-E-caffeoyl-O-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | OH | OH | H | Api | H | H | Caffeoyl | L. contortus | Cytotoxity | [29] |
| 45 | β-(3,4-Dihydroxyphenyl) ethyl-4-O-E-caffeoyl-O-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | OH | OH | H | Api | H | Caffeoyl | H | L. contortus | - b | [29] |
| 46 | β-(3,4-Dihydroxyphenyl) ethyl-3-O-E-caffeoyl-O-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | OH | OH | H | Api | Caffeoyl | H | H | L. contortus | Cytotoxity | [29] |
| 47 | β-(4-Hydroxyphenyl) ethyl-3-O-E-caffeoyl-O-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside | H | OH | H | Api | Caffeoyl | H | H | L. contortus | - b | [29] |
| 48 | Lagotiside A | OH | OH | H | H | 4-O-CH3-Xyl | Caffeoyl | H | Lagotis brevituba | - a | [30] |
| 49 | Yulanoside A | OH | OH | H | Rha | Rha | Caffeoyl | Glc(1→4)Glc | Magnolia salicifolia | - a | [31] |
| 50 | Yulanoside B | OH | OH | H | H | Rha | Caffeoyl | Glc(1→4)Glc | M. salicifolia | - a | [31] |
| 51 | 2′-Rhamnoechinacoside | OH | OH | H | Rha | Rha | Caffeoyl | Glc | M. salicifolia | α-Glucosidase inhibitory effect and cytotoxicity | [31,32] |
| 52 | Magnoloside D | OH | OH | H | Rha | H | H | Caffeoyl | M. officinalis | Antioxidant activity, α-glucosidase inhibitory effect and cytotoxicity | [32,33] |
| 53 | Magnoloside E | OH | OH | H | Api | H | H | Caffeoyl | M. officinalis | Antioxidant activity, α-glucosidase inhibitory effect and cytotoxicity | [32,33] |
| 54 | Magnoloside F | OH | OH | H | Rha | H | Caffeoyl | Glc | M. officinalis | α-Glucosidase inhibitory effect and cytotoxicity | [32] |
| 55 | Magnoloside G | OH | OH | H | Api | H | Caffeoyl | Glc | M. officinalis | Cytotoxicity | [32] |
| 56 | Magnoloside H | OH | OH | H | Api | Caffeoyl | H | Glc | M. officinalis | α-Glucosidase inhibitory effect and cytotoxicity | [32] |
| 57 | Magnoloside I | OH | OH | H | Api | Coumaroyl | H | Glc | M. officinalis | α-Glucosidase inhibitory effect | [32] |
| 58 | Magnoloside J | OH | OCH3 | H | Rha | Caffeoyl | H | Glc | M. officinalis | Cytotoxicity | [32] |
| 59 | Magnoloside K | OH | OH | H | Rha | Feruloyl | H | Glc | M. officinalis | α-Glucosidase inhibitory effect and cytotoxicity | [32] |
| 60 | Magnoloside L | OH | OH | H | Api | Caffeoyl | H | H | M. officinalis | Cytotoxicity | [32] |
| 61 | Magnoloside M | OH | OH | H | Rha | H | Caffeoyl | H | M. officinalis | - a | [32] |
| 62 | Magnoloside N | OH | O-Glc | H | Rha | Caffeoyl | H | Glc | M. officinalis | - a | [32] |
| 63 | Magnoloside O | OH | OH | H | H | H | H | Glc(1→4)Rha(1→4)-Syringoyl | M. officinalis | Cytotoxicity | [32] |
| 64 | Magnoloside P | OH | OH | H | H | H | H | Glc(1→4)Rha(1→4)-Vanilloyl | M. officinalis | Cytotoxicity | [32] |
| 65 | Savaside A | OH | OH | OH | Rha | H | H | Caffeoyl | Monochasma savatieri | Anticomplement activity | [34] |
| 66 | Savaside B | OH | OH | OH | Rha | H | Caffeoyl | H | M. savatieri | Anticomplement activity | [34] |
| 67 | Savaside C | OH | OH | OH | Rha | H | Feruloyl | H | M. savatieri | Anticomplement activity | [34] |
| 68 | Savaside D | OH | OH | OH | Rha | H | H | Coumaroyl | M. savatieri | Anticomplement activity | [34] |
| 69 | Savaside E | OH | OH | OH | Rha | H | H | Feruloyl | M. savatieri | Anticomplement activity | [34] |
| 70 | Rashomoside A | OH | OH | H | H | Xyl | Caffeoyl | Glc | Meehania urticifolia | - b | [35] |
| 71 | Tazettoside D | H | OCH3 | H | H | H | H | Glc | Narcissus tazetta var. chinensis | Melanogenesis inhibitory activity | [36] |
| 72 | 3-Hydroxy-4-methoxy-β-phenylethoxy-O-[2,3-di-acetyl-α-L-rhamnopyranosyl-(1→3)]-4-O-cis-feruloyl-[β-d-apiofuranosyl-(1→6)]-β-d-glucopyranoside | OH | OCH3 | H | H | 2,3-di-O-Acetyl-Rha | cis-Feruloyl | Api | Phlomis umbrosa | - a | [37] |
| 73 | 3′′′-Acetyl-O-betonyoside D | OH | OCH3 | H | H | 3-O-Acetyl-Rha | Feruloyl | Api | P. umbrosa | Cytotoxic activity | [38] |
| 74 | 2′′′, 3′′′-Diacetyl-O-betonyoside D | OH | OCH3 | H | H | 2,3-di-O-Acetyl-Rha | Feruloyl | Api | P. umbrosa | Cytotoxic activity | [38] |
| 75 | 3′′′,4′′′-Diacetyl-O-betonyoside D | OH | OCH3 | H | H | 3,4-di-O-Acetyl-Rha | Feruloyl | Api | P. umbrosa | Cytotoxic activity | [38] |
| 76 | Stewartiiside | OH | OH | H | H | Api(1→4)Rha | Caffeoyl | Rha | P. stewartii | α-Glucosidase inhibitory activity | [39] |
| 77 | 2-(3-Hydroxy-4-methoxyphenyl) ethanol 1-O-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside] | OH | OCH3 | H | Rha | H | H | H | Plantago depressa | - a | [40] |
| 78 | 2-(3,4-Dihydroxyphenyl) ethyl 3-O-β-d-allopyranosyl-6-O-caffeoyl-β-d-glucopyranoside | OH | OH | H | H | All | H | Caffeoyl | P. asiatica | Antioxidative effect | [41] |
| 79 | Isocassifolioside | OH | OH | H | Rha | Rha | H | Caffeoyl | Ruellia tuberosa | Antioxidant activity | [42] |
| 80 | Lavandulifolioside B | OCH3 | OH | H | H | Ara(1→2)Rha | 4-O-CH3-Feruloyl | H | Stachys lavandulifolia | - b | [43] |
| 81 | Poliumoside B | OH | OH | H | H | Ara(1→2)Rha | Caffeoyl | Rha | Teucrium polium | Antioxidant activity | [44] |
| 82 | 1-(3,4-Dihydroxyphenylethyl)-O-α-l-lyxopyranosyl-(1→2)-α-l-hamnopyranosyl-(1→3)-6-O-transferuloyl-β-d-glucopyranoside | OH | OH | H | H | Lyx(1→2)Rha | H | Feruloyl | T. chamaedris | Antioxidant activity | [45] |
| 83 | Chionoside A | OH | OH | H | Ara | Glc | Feruloyl | H | Veronica thomsonii | - a | [46] |
| 84 | Chionoside B | OH | OCH3 | H | Ara | Glc | Feruloyl | H | V. thomsonii | - a | [46] |
| 85 | Chionoside C | OH | OH | H | Ara | 6-O-Feruloyl-Glc | Caffeoyl | H | V. thomsonii | - a | [46] |
| 86 | Chionoside D | OH | OH | H | Ara | Glc | Caffeoyl | Glc | V. thomsonii | - a | [46] |
| 87 | Chionoside E | OH | OH | H | Ara | Glc | Feruloyl | Glc | V. thomsonii | - a | [46] |
| 88 | Chionoside F | OH | OH | H | Ara | Glc | Caffeoyl | Rha | V. thomsonii | - a | [46] |
| 89 | Chionoside G | OH | OCH3 | H | Glc | Glc | Caffeoyl | H | V. pulvinaris | - a | [46] |
| 90 | Chionoside I | OH | OCH3 | H | Glc | Glc | Feruloyl | H | V. thomsonii and V. pulvinaris | - a | [46] |
| 91 | Isochionoside J | OH | OH | H | H | Glc(1→2)Glc | H | Caffeoyl | V. thomsonii | - a | [46] |
| 92 | Isoaragoside | OH | OH | H | Ara | Glc | H | Caffeoyl | V. thomsonii | - a | [46] |
| 93 | Isochionoside K | OH | OCH3 | H | Ara | Glc | H | Caffeoyl | V. thomsonii | - a | [46] |
| 94 | Isochionoside A | OH | OH | H | Ara | Glc | H | Feruloyl | V. thomsonii | - a | [46] |
| 95 | Isochionoside G | OH | OCH3 | H | Glc | Glc | H | Caffeoyl | V. pulvinaris | - a | [46] |
| 96 | Isochionoside I | OH | OCH3 | H | Glc | Glc | H | Feruloyl | V. thomsonii and V. pulvinaris | - a | [46] |
| 97 | Helioside A | OH | OH | H | Ara | Glc | Caffeoyl | Xyl | V. lavaudiana | - a | [47] |
| 98 | Helioside B | OH | OH | H | Ara | 6-O-Caffeoyl-Glc | Caffeoyl | Xyl | V. lavaudiana | - a | [47] |
| 99 | Helioside C | OH | OH | H | Ara | Glc | Feruloyl | Xyl | V. lavaudiana | - a | [47] |
| 100 | Helioside D | OH | OH | H | Ara | 6-O-Coumaroyl-Glc | Caffeoyl | H | V. raoulii | - a | [48] |
| 101 | Helioside E | OH | OH | H | Ara | 6-O-Caffeoyl-Glc | Caffeoyl | H | V. raoulii | - a | [48] |
| 102 | Helioside F | OH | OH | H | Xyl | Glc | Caffeoyl | Glc | V. hulkeana | - a | [48] |
| No. | Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|
| 117 | Echinacoside | OH | OH | H | H | Rha | Caffeoyl | Glc |
| 118 | Pedicularioside A | OH | OH | H | H | Api | Caffeoyl | Rha |
| 119 | Leucosceptoside A | OH | OH | H | H | Rha | Feruloyl | H |
| 120 | Isoacteoside | OH | OH | H | H | Rha | H | Caffeoyl |
| 121 | Acteoside (Verbascoside) | OH | OH | H | H | Rha | Caffeoyl | H |
| 122 | Arenariside | OH | OH | H | H | Rha | Caffeoyl | Xyl |
| 123 | Salidroside | H | OH | H | H | H | H | H |
| 124 | Forsythoside (Forsythiaside/Forsythoside A) | OH | OH | H | H | H | Caffeoyl | Rha |
| 125 | Forsythoside B | OH | OH | H | H | Rha | Caffeoyl | Api |
| 126 | Leucosceptoside B | OH | OCH3 | H | H | Rha | Feruloyl | Api |
| 127 | Calceorioside A | OH | OH | H | H | H | Caffeoyl | H |
| 128 | Poliumoside | OH | OH | H | H | Rha | Caffeoyl | Rha |
| 129 | Alyssonoside | OH | OH | H | H | Rha | Feruloyl | Api |
| 130 | Brandioside | OH | OH | H | Acetyl | Rha | Caffeoyl | Rha |
| 131 | Isocampneoside II | OH | OH | OH | H | Rha | H | Caffeoyl |
| 132 | 6-O-Acetylacteoside | OH | OH | H | H | Rha | Caffeoyl | Acetyl |
| 133 | 4′′′-O-Acetylacteoside | OH | OH | H | H | 4-O-Acetyl-Rha | Caffeoyl | H |
| 134 | Decaffeoylacteoside | OH | OH | H | H | Rha | H | H |
| 135 | Teucrioside | OH | OH | H | H | Lyx(1→2)Rha | Caffeoyl | H |
| 136 | Lamiuside A | OH | OH | H | H | Gal(1→2)Rha | Caffeoyl | H |
| 137 | 2′-Acetylacteoside | OH | OH | H | Acetyl | Rha | Caffeoyl | H |
| 138 | Plantamajoside | OH | OH | H | H | Glc | Caffeoyl | H |
| 139 | Tubuloside B | OH | OH | H | Acetyl | Rha | H | Caffeoyl |
| 140 | Tyrosol galactoside | H | OH | H | H | H | H | H |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Yang, B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules 2016, 21, 991. https://doi.org/10.3390/molecules21080991
Xue Z, Yang B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules. 2016; 21(8):991. https://doi.org/10.3390/molecules21080991
Chicago/Turabian StyleXue, Zhenzhen, and Bin Yang. 2016. "Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics" Molecules 21, no. 8: 991. https://doi.org/10.3390/molecules21080991
APA StyleXue, Z., & Yang, B. (2016). Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules, 21(8), 991. https://doi.org/10.3390/molecules21080991