Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Partial Chemical Characterization of Lentinula edodes
2.2. Lentinula edodes Attenuated Inflammatory Stress in Stimulated Keratinocytes
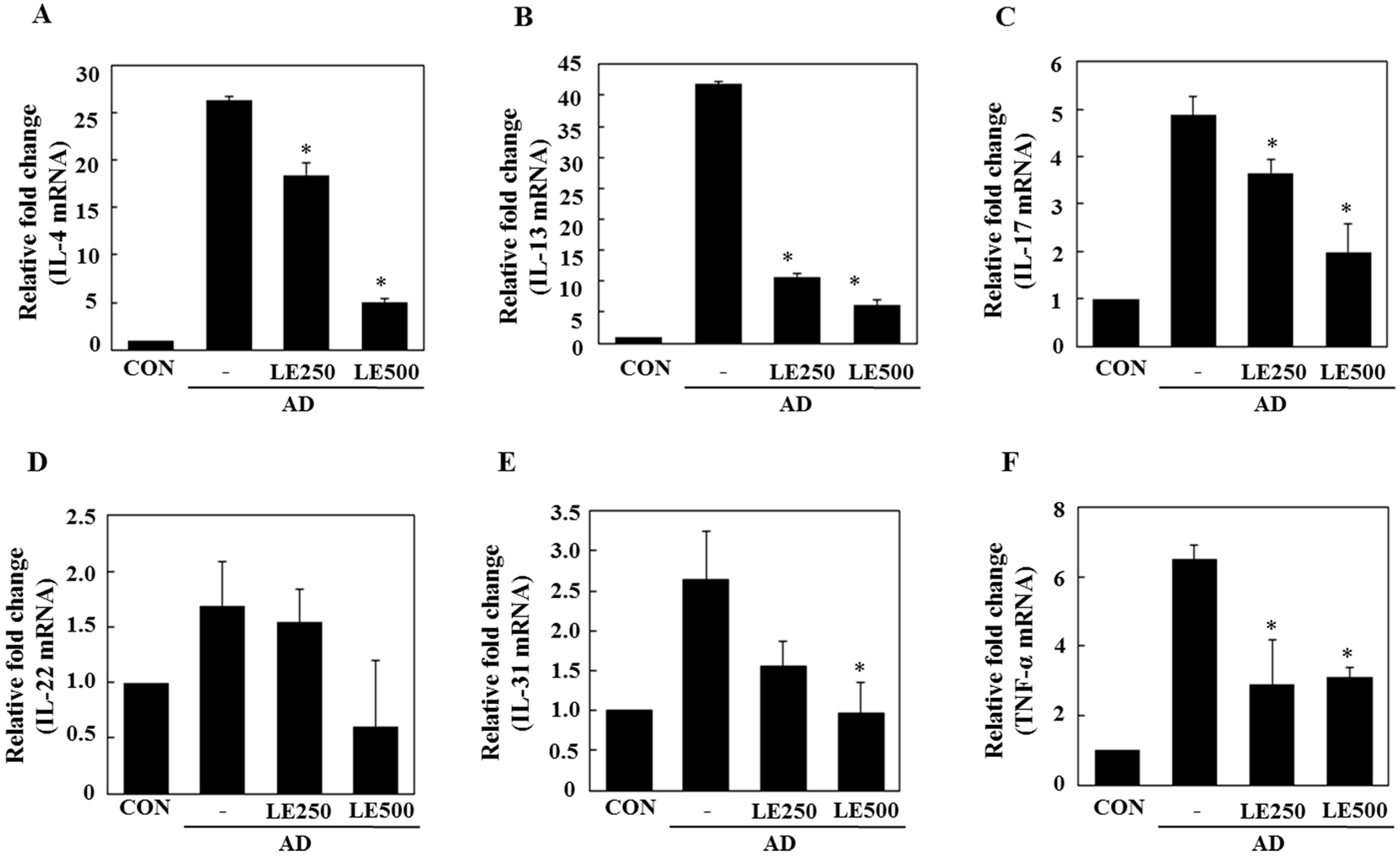
2.3. Lentinula edodes Supressed Inflammatory Cytokines in a Murine Model
3. Materials and Methods
3.1. Materials
3.2. Analysis of Polysaccharides and Monosaccharides
3.3. Analysis of Protein Content and Amino Acids
3.4. Phytochemical Content
3.5. FT-IR Spectroscopy
3.6. Cell Culture
3.7. Animals
3.8. Induction of AD Lesions in the Ear
3.9. Histological Observations
3.10. Analysis of mRNA Expression
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gry, J.; Andersson, C.; Kruger, L.; Lyran, B.; Jensvoll, L.; Matilainen, N.; Nurttila, A.; Olafsson, G.; Fabech, B. TemaNord; Nordic Council of Ministers: Copenhagen, Denmark, 2012; p. 81. [Google Scholar]
- Yang, Z. The Divine Farmer’s Materia Medica: A Translation of the Shen Nong Ben Cao; Blue Poppy Press: Boulder, CO, USA, 1998; p. 121. [Google Scholar]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Chang, S.T.; Buswell, J.A. Medicinal mushrooms—A prominent source of nutraceuticals for the 21st century. Curr. Top. Nutraceutical Res. 2003, 1, 257–280. [Google Scholar]
- Wani, B.A.; Bodha, R.H.; Wani, A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar]
- Cheung, P.C.K. Nutritional value and health benefits of mushrooms. In Mushrooms as Functional Foods; John Wiley and Sons, Inc.: New York, NY, USA, 2008; p. 71. [Google Scholar]
- Fan, L.; Pan, H.; Soccol, A.T.; Pandey, A.; Soccol, C.R. Advances in mushroom research in the last decade. Food Technol. Biotechnol. 2006, 44, 303–311. [Google Scholar]
- Lindequist, U.; Niedermeyer, T.H.S.; Julich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [PubMed]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hao, M.; Nan, H.; Jeff, I.; Zhou, Y.; Gao, Y. Relationship of chemical composition and cytotoxicity of water-soluble polysaccharides from Lentinus edodes fruiting bodies. Pak. J. Pharm. Sci. 2015, 28, 1069–1074. [Google Scholar] [PubMed]
- Kabir, Y.; Yamaguchi, M.; Kimura, S. Effect of shiitake (Lentinus edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 1987, 33, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C. Medicinal Value of Lentinus edodes (Berk.) Sing. (Agaricomycetideae). A Literature Review. Int. J. Med. Mushrooms 2000, 2, 16. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Chong, Y.L.; Mahmood, A.A.; Indran, M.; Abdullah, N.; Vikineswary, S. Lentinula edodes (Shiitake) mushroom extract protects against hydrogen peroxide induced cytotoxicity in peripheral blood mononuclear cells. Indian J. Biochem. Biophys. 2009, 46, 161–165. [Google Scholar] [PubMed]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Aalto-Korte, K.; Susitaival, P.; KaMinska, R.; Mäkinen-Kiljunen, S. Occupational protein contact dermatitis from shiitake mushroom and demonstration of shiitake-specific immunoglobulin E. Contact Derm. 2005, 53, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Curnow, P.; Tam, M. Contact dermatitis to Shiitake mushroom. Australas. J. Dermatol. 2003, 44, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Obama, K.; Aoyama, K.; Ueda, T.; Xu, B.H.; Li, Q.; Huang, J.; Kitano, T.; Inaoka, T. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermat. 1992, 26, 228–233. [Google Scholar] [CrossRef]
- Tarvainen, K.; Salonen, J.P.; Kanerva, L.; Estlander, T.; Keskinen, H.; Rantanen, T. Allergy and toxicodermia from shiitake mushrooms. J. Am. Acad. Dermatol. 1991, 24, 64–66. [Google Scholar] [CrossRef]
- Nakamura, T. Shiitake (Lentinus edodes) dermatitis. Contact Dermat. 1992, 27, 65–70. [Google Scholar] [CrossRef]
- Haas, N.; Vogt, R.; Sterry, W. Shiitake dermatitis: Flagellate dermatitis after eating mushrooms. Hautarzt 2001, 52, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Adriano, A.R.; Acosta, M.L.; Azulay, D.R.; Quiroz, C.D.; Talarico, S.R. Shiitake dermatitis: The first case reported in Brazil. An. Bras. Dermatol. 2013, 88, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Boels, D.; Landreau, A.; Bruneau, C.; Garnier, R.; Pulce, C.; Labadie, M.; de Haro, L.; Harry, P. Shiitake dermatitis recorded by French Poison Control Centers—New case series with clinical observations. Clin. Toxicol. 2014, 52, 625–628. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Ross, C.; Wayte, J. Shiitake dermatitis: The tale of an under-recognised, undercooked fungus. Med. J. Aust. 2016, 204, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H. Antiallergy Agents Containing Basidiomycota Extracts. Japan Patent JP 2004067567, 4 March 2004. [Google Scholar]
- Shimizu, M. Medicinal Composition Comprising Dried Powder or Water Extract of Grifola frondosa for Treating Chronic Diseases. Japan Patent JP 06072889, 15 March 1994. [Google Scholar]
- Frankell, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Becker, S.P. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G.R. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem. 2014, 15, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1986, 44, 7–89. [Google Scholar] [PubMed]
- Choi, J.K.; Kim, S.H. Inhibitory effect of galangin on atopic dermatitis-like skin lesions. Food Chem. Toxicol. 2014, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1562. [Google Scholar]
- Schmid-Grendelmeier, P.; Simon, D.; Simon, H.U.; Akdis, C.A.; Wuthrich, B. Epidemiology, clinical features, and immunology of the “intrinsic” (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy 2001, 56, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Dokmeci, E.; Herrick, C.A. The immune system and atopic dermatitis. Semin. Cutan. Med. Surg. 2008, 27, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Bhan, A.K.; Schneeberger, E.E.; Geha, R.S. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J. Allergy Clin. Immunol. 1983, 71, 47–56. [Google Scholar] [CrossRef]
- Albanesi, C.; Cavani, A.; Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-α. J. Immunol. 1999, 162, 494–502. [Google Scholar] [PubMed]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Stabauer, M.; Boehner, A.; Eyerich, S.; Carbone, T.; Traidl-Hoffmann, C.; Schmidt-Weber, C.B.; Cavani, A.; Ring, J.; Hein, R.; Eyerich, K. Differential in situ expression of IL-17 in skin diseases. Eur. J. Dermatol. 2012, 22, 781–784. [Google Scholar] [PubMed]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Leung, D.Y.; Molet, S.; Boguniewicz, M.; Taha, R.; Christodoulopoulos, P.; Fukuda, T.; Elias, J.A.; Hamid, Q.A. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003, 111, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Bernard, F.X.; Garcia, M.; Gurney, A.L.; Lecron, J.C.; Morel, F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Zaba, L.C.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Kikuchi, T.; Ramon, M.; Bergman, R.; Krueger, J.G.; Guttman-Yassky, E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009, 123, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Neis, M.M.; Peters, B.; Dreuw, A.; Wenzel, J.; Bieber, T.; Mauch, C.; Krieg, T.; Stanzel, S.; Heinrich, P.C.; Merk, H.F.; et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006, 118, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hwang, Y.H.; Kim, M.K.; Hong, G.E.; Lee, H.J.; Nagappan, A.; Yumnam, S.; Kim, E.H.; Heo, J.D.; Lee, S.J.; et al. Functional polysaccharides from Grifola frondosa aqueous extract inhibit atopic dermatitis-like skin lesions in NC/Nga mice. Biosci. Biotechnol. Biochem. 2015, 79, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ukawa, Y.; Izumi, Y.; Ohbuchi, T.; Takahashi, T.; Ikemizu, S.; Kojima, Y. Oral administration of the extract from Hatakeshimeji (Lyophyllum decastes sing.) mushroom inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Sci. Vitaminol. 2007, 53, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, M.; Gilles, K.A.; Hamitton, J.K.; Reders, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruits. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, E.K.; Choi, E.J. High-intensity swimming exercise increases dust mite extract and 1-chloro-2,4-dinitrobenzene-derived atopic dermatitis in BALB/c mice. Inflammation 2014, 37, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.





| Components | Content | |
|---|---|---|
| Total carbohydrates (μg/g) | 151.43 ± 1.05 | |
| Monosaccharides (μg/mL) | Arabinose | 2.57 ± 0.17 |
| Galactose | 1.83 ± 0.19 | |
| Glucose | 127.78 ± 1.32 | |
| Mannose | 4.27 ± 0.53 | |
| Xylose | 2.5 ± 0.51 | |
| Total protein (μg/g) | 205.17 ± 1.44 | |
| Amino acids (g/100 g) | Asp | 0.4725 |
| Thr | 0.4435 | |
| Ser | 0.3789 | |
| Glu | 2.8103 | |
| Pro | 0.1789 | |
| Gly | 0.3781 | |
| Ala | 0.6621 | |
| Val | 0.3696 | |
| Ile | 0.0963 | |
| Leu | 0.1554 | |
| Tyr | 0.0711 | |
| Phe | 0.1393 | |
| Lys | 0.4127 | |
| His | 0.1611 | |
| Arg | 0.3783 | |
| Cys | 0.5109 | |
| Met | 0.0284 | |
| Phytochemicals | Polyphenols (mg/g) | 6.12 ± 0.04 |
| Flavonoids (mg/g) | 1.76 ± 0.22 | |
| β-Carotene (μg/g) | 28.75 ± 0.25 | |
| Lycopene (μg/g) | 5.25 ± 0.04 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.-J.; Park, Z.-Y.; Kim, E.-K. Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo. Molecules 2016, 21, 993. https://doi.org/10.3390/molecules21080993
Choi E-J, Park Z-Y, Kim E-K. Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo. Molecules. 2016; 21(8):993. https://doi.org/10.3390/molecules21080993
Chicago/Turabian StyleChoi, Eun-Ju, Zee-Yong Park, and Eun-Kyung Kim. 2016. "Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo" Molecules 21, no. 8: 993. https://doi.org/10.3390/molecules21080993
APA StyleChoi, E.-J., Park, Z.-Y., & Kim, E.-K. (2016). Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo. Molecules, 21(8), 993. https://doi.org/10.3390/molecules21080993