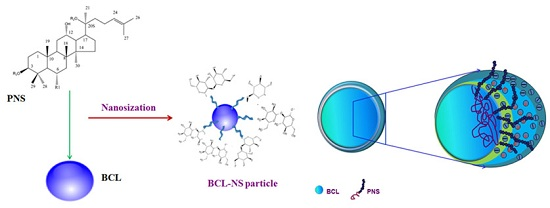
Panax Notoginseng Saponins as a Novel Nature Stabilizer for Poorly Soluble Drug Nanocrystals: A Case Study with Baicalein
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influences of PNS on the Dispersion Efficiency and Stability of BCL-NS during Homogenization
2.2. Effect of PNS with or without Other Cryoprotectants on Redispersibility of BCL-NC during Freeze-Drying Process
2.3. Effect of PNS with or without Other Matrix Formers on Redispersibility of BCL-NC during Spray-Drying Process
3. Experiment Section
3.1. Materials
3.2. Production of BCL-NS
3.3. Characterization of BCL-NS
3.3.1. Particle Size Measurements
3.3.2. Zeta Potential Assay
3.3.3. Stability Index (SI)
3.3.4. Determination of Surface Tension
3.4. Conversion BCL-NS into BCL-NC via Freeze-Drying
3.4.1. Effect of PNS with or without Other Cryoprotectants on Redispersibility of BCL-NC during Freezing Process
3.4.2. Roles of PNS with or without Other Cryoprotectants on Redispersibility of BCL-NC during Lyophilization Process
3.4.3. Conversion BCL-NS into BCL-NC via Spray-Drying
3.5. Redispersibility (RDI) Index of BCL-NC
3.6. Scanning Electron Microscope (SEM) of BCL-NC
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of Nanocrystals-Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Kayser, O.; Müller, R.H. Nanosuspensions as a new approach for the formulation for the poorly soluble drug tarazepide. Int. J. Pharm. 2000, 196, 161–164. [Google Scholar] [CrossRef]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, M.V.; Popescu, C. Conversion of nanosuspensions into dry powders by spray drying: A case study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J. Effective polymeric dispersants for vacuum, convection and freeze drying of drug nanosuspensions. Int. J. Pharm. 2010, 397, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.E.; Bose, S. Spray granulation: Importance of process parameters on in vitro and in vivo behavior of dried nanosuspensions. Eur. J. Pharm. Biopharm. 2013, 85, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.F.; Li, G.; Dan, J.X.; Wu, Z.F.; Wang, C.H.; Zhu, W.F.; Yang, M. Study on formability of solid nanosuspensions during solidification: II novel roles of freezing stress and cryoprotectant property. Int. J. Pharm. 2014, 475, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Ji, Y.Y.; Kwak, H.S.; Nam, B.U.; Lee, J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 2005, 5, 472–474. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Van Humbeeck, J.; Augustijns, P.; van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.Y.; Park, C.H. Characteristics of polymers enabling nanocomminution of water-insoluble drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.F.; Wang, C.H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef] [PubMed]
- San Martin, R.; Briones, R. Industrial uses and sustainable supply of Quillaja saponaria (Rosaceae) saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar] [CrossRef]
- Guclu-Ustundag, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocolloids 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Yang, Y.; Leser, M.E.; Sher, A.A.; Mcclements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocolloids 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Chou, C.C.; Pan, S.L.; Teng, C.M.; Guh, J.H. Pharmacological evaluation of several major ingredients of Chinese herbal medicines in human hepatoma Hep3B cells. Eur. J. Pharm. Sci. 2003, 19, 403–412. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.T.; Pu, S.P. Zinc coupling potentiates anti-HIV-1 activity of baicalin. Biochem. Biophys. Res. Commun. 2004, 324, 605–610. [Google Scholar]
- Hong, T.; Jin, G.B.; Cho, S.; Cyong, J.C. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002, 68, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Kimura, Y.; Odani, T.; Tani, T.; Namba, K. Studies on Scutellaria radix. Part 2: The antibacterial substance. Planta Med. 1981, 43, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Bae, S.M.; Kim, K.J.; Kim, W.; Chung, S.I.; Yoon, Y. β-Catenin mediates the anti-adipogenic effect of baicalin. Biochem. Biophys. Res. Commun. 2010, 398, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Ma, Y.; Yue, P.; Xie, Y.; Zheng, Q.; Hu, P.; Zhu, W.; Yang, M. Microcrystalline cellulose-carboxymethyl cellulose sodium as an effective dispersant for drug nanocrystals: A case study. Carbohydr. Polym. 2016, 136, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.F.; Wang, C.H.; Dan, J.X.; Liu, W.; Yang, M. The importance of solidification stress on the redispersibility of solid nanocrystals loaded with harmine. Int. J. Pharm. 2015, 475, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.








| Dispersants | BCL-NS/PNS | BCL-NS/PVPK30 | BCL-NS/Tween 80 | BCL-NS/HPMC |
|---|---|---|---|---|
| D50 (µm) | 0.156 ± 0.012 | 0.145 ± 0.006 | 0.144 ± 0.004 | 0.149 ± 0.005 |
| Span | 3.145 ± 0.013 | 4.250 ± 0.011 | 4.264 ± 0.007 | 5.198 ± 0.012 |
| SI | 0.969 ± 0.012 | 1.021 ± 0.006 | 1.056 ± 0.004 | 1.443 ± 0.005 |
| ZP (mV) | −40.1 ± 1.6 | −31.7 ± 2.3 | −33.4 ± 1.4 | −29.1 ± 3.1 |
| Stabilizer Concentrations (%, w/w) | Physicochemical Property | |||||
|---|---|---|---|---|---|---|
| Viscosity (mPa s) | Surface Tension (mN/m) | |||||
| 50 | 25 | 10 | 50 | 25 | 10 | |
| PNS | 5.02 ± 0.29 | 4.91 ± 0.17 | 4.49 ± 0.11 | 41.69 ± 0.32 | 41.92 ± 0.22 | 44.17 ± 0.28 |
| PVPK30 | 6.10 ± 0.51 | 5.43 ± 0.37 | 5.01 ± 0.21 | 42.18 ± 0.56 | 45.06 ± 0.76 | 47.40 ± 0.84 |
| Tween-80 | 5.08 ± 0.35 | 4.73 ± 0.29 | 4.61 ± 0.32 | 40.60 ± 0.55 | 40.71 ± 0.64 | 42.49 ± 0.71 |
| HPMC | 8.89 ± 0.32 | 8.23 ± 0.28 | 5.01 ± 0.41 | 42.15 ± 0.94 | 42.57 ± 1.02 | 43.04 ± 0.65 |
| Temperature Strength | “Conservative” | “Moderate” | “Aggressive” |
|---|---|---|---|
| Freezing | −20 °C for 12 h | −80 °C for 6 h | −196 °C for 2 h |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Ma, Y.; Xu, J.; Liu, Y.; Yue, P.; Zheng, Q.; Hu, P.; Yang, M. Panax Notoginseng Saponins as a Novel Nature Stabilizer for Poorly Soluble Drug Nanocrystals: A Case Study with Baicalein. Molecules 2016, 21, 1149. https://doi.org/10.3390/molecules21091149
Xie Y, Ma Y, Xu J, Liu Y, Yue P, Zheng Q, Hu P, Yang M. Panax Notoginseng Saponins as a Novel Nature Stabilizer for Poorly Soluble Drug Nanocrystals: A Case Study with Baicalein. Molecules. 2016; 21(9):1149. https://doi.org/10.3390/molecules21091149
Chicago/Turabian StyleXie, Yuanbiao, Yueqin Ma, Junnan Xu, Yang Liu, Pengfei Yue, Qin Zheng, Pengyi Hu, and Ming Yang. 2016. "Panax Notoginseng Saponins as a Novel Nature Stabilizer for Poorly Soluble Drug Nanocrystals: A Case Study with Baicalein" Molecules 21, no. 9: 1149. https://doi.org/10.3390/molecules21091149
APA StyleXie, Y., Ma, Y., Xu, J., Liu, Y., Yue, P., Zheng, Q., Hu, P., & Yang, M. (2016). Panax Notoginseng Saponins as a Novel Nature Stabilizer for Poorly Soluble Drug Nanocrystals: A Case Study with Baicalein. Molecules, 21(9), 1149. https://doi.org/10.3390/molecules21091149