Chemical and Sensory Evaluation of Silicone and Polylactic Acid-Based Remedial Treatments for Elevated Methoxypyrazine Levels in Wine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analyses Results
2.1.1. Methoxypyrazines
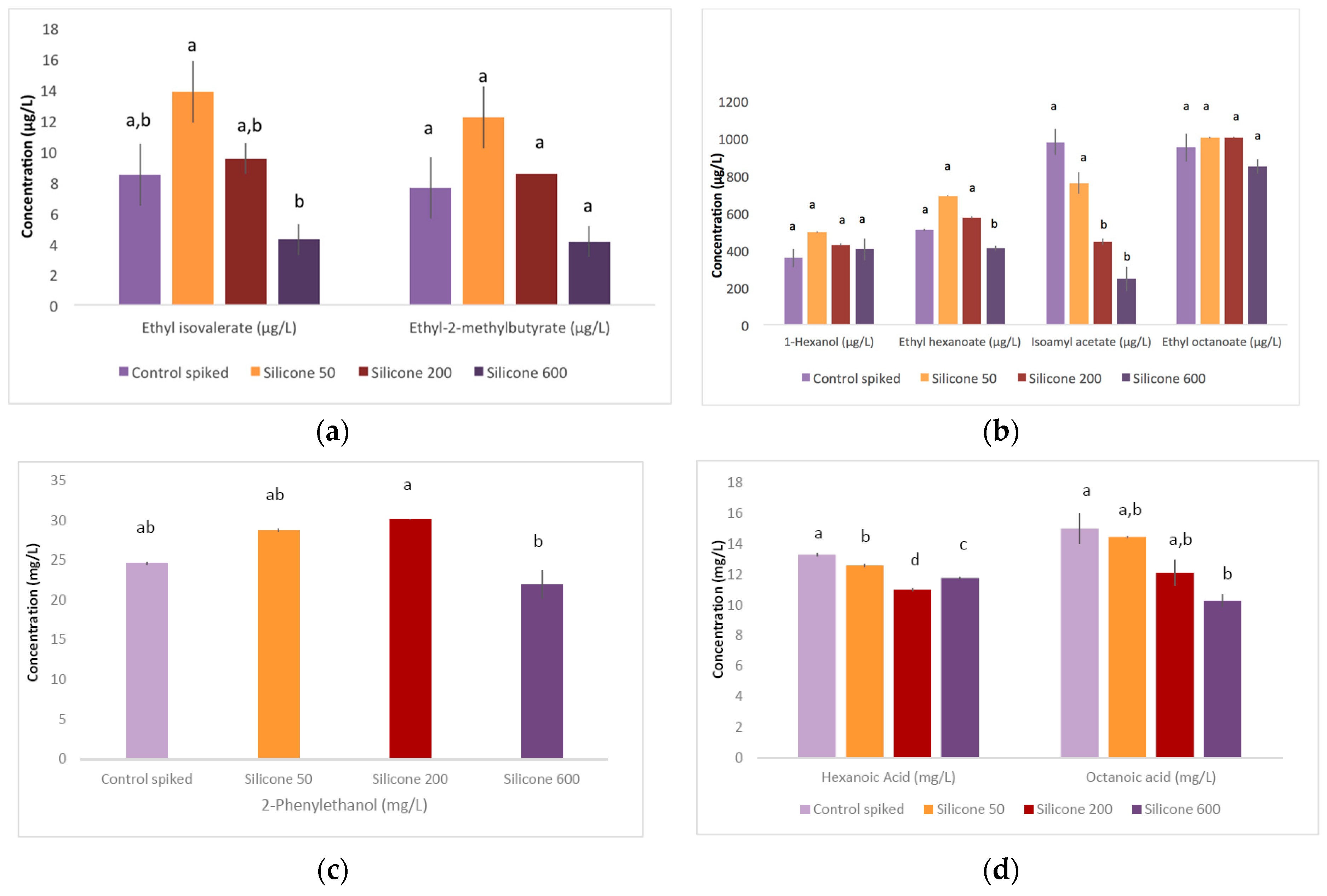
2.1.2. Other Volatile Aroma Compounds
2.1.3. Color and Lactic Acid Analyses
2.2. Sensory Analysis
3. Experimental Section
3.1. Wines
3.2. Treatments
3.3. Analysis Methods
3.3.1. Methoxypyrazine Analysis
3.3.2. Analysis of Volatile Aroma Compounds (VOCs)
3.3.3. Preparation of Standards of Volatile Aroma Compounds (VOCs)
3.3.4. Preparation of Standards of Volatile Fatty Acids
3.3.5. Sample Preparation
3.3.6. Head Space-Solid Phase Micro-Extraction-Gas Chromatography (HS-SPME-GC-MS) Parameters for VOCs
3.3.7. Head Space Solid Phase Micro-extraction-Gas-Chromatography-Mass Spectrometry (HS-SPME-GC-MS) Parameters for Volatile Fatty Acids
3.3.8. Data Processing
3.3.9. Other Methods
3.3.10. Sensory Analysis
3.3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Botezatu, A.; Pickering, G.J.; Kotseridis, Y. Development of a rapid method for the quantitative analysis of four methoxypyrazines in white and red wine using multi-dimensional Gas Chromatography-Mass Spectrometry. Food Chem. 2014, 160, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. A Global Survey of Methoxypyrazines in Wine. J. Food Agric. Environ. 2016, 14, 24–29. [Google Scholar]
- Pickering, G.; Lin, J.; Riesen, R.; Reynolds, A.; Brindle, I.; Soleas, G. Influence of Harmonia axyridis on the sensory properties of white and red wine. Am. J. Enol. Vitic. 2004, 55, 153–159. [Google Scholar]
- Botezatu, A.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. Occurrence and contribution of alkyl methoxypyrazines in wine tainted by Harmonia axyridis and Coccinella septempunctata. J. Sci. Food Agric. 2013, 93, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Kögel, S.; Botezatu, A.; Hoffmann, C.; Pickering, G. Methoxypyrazine composition of Coccinellidae tainted Riesling and Pinot noir wine from Germany. J. Sci. Food Agric. 2015, 95, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.J.; Karthik, A.; Inglis, D.; Sears, M.; Ker, K. Determination of Ortho-and Retronasal Detection Thresholds for 2-Isopropyl-3-Methoxypyrazine in Wine. J. Food Sci. 2007, 72, S468–S472. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Glemser, E.J.; Hallett, R.; Inglis, D.; McFadden-Smith, W.; Ker, K. Good Bugs Gone Bad: Coccinellidae, Sustainability and Wine. Sustainability Today; WIT Press: Southampton, UK, 2012; pp. 239–251. [Google Scholar]
- Hashizume, K.; Samuta, T. Grape maturity and light exposure affect berry methoxypyrazine concentration. Am. J. Enol. Vitic. 1999, 50, 194–198. [Google Scholar]
- Belancic, A.; Agosin, E. Methoxypyrazines in Grapes and Wines of Vitis. vinifera cv. Carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- De Boubée, D.R.; Cumsille, A.M.; Pons, M.; Dubourdieu, D. Location of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon grape bunches and its extractability during vinification. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Pickering, G.J.; Lin, J.; Reynolds, A.; Soleas, G.; Riesen, R. The evaluation of remedial treatments for wine affected by Harmonia axyridis. Int. J. Food Sci. Technol. 2006, 41, 77–86. [Google Scholar] [CrossRef]
- Blake, A.; Kotseridis, Y.; Brindle, I.D.; Inglis, D.; Pickering, G.J. Effect of light and temperature on 3-alkyl-2-methoxypyrazine concentration and other impact odorants of Riesling and Cabernet Franc wine during bottle ageing. Food Chem. 2010, 119, 935–944. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of severity of post-flowering leaf removal on berry growth and composition of three red Vitis vinifera L. cultivars grown under semiarid conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Koegel, S.; Gross, J.; Hoffmann, C. Sensory detection thresholds of “ladybird taint” in ‘Riesling’ and ‘Pinot Noir’ under different fermentation and processing conditions. Vitis 2012, 51, 27–32. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Inglis, D.; Brindle, I.D.; Sears, M.; Beh, A.L. Yeast strain affects 3-isopropyl-2-methoxypyrazine concentration and sensory profile in Cabernet Sauvignon wine. Aust. J. Grape Wine Res. 2008, 14, 230–237. [Google Scholar] [CrossRef]
- Pickering, G.J.; Blake, A.J.; Soleas, G.J.; Inglis, D.L. Remediation of wine with elevated concentrations of 3-alkyl-2-methoxypyrazines using cork and synthetic closures. J. Food Agric. Environ. 2010, 8, 97–101. [Google Scholar]
- Ryona, I.; Reinhardt, J.; Sacks, G.L. Treatment of grape juice or must with silicone reduces 3-alkyl-2-methoxyprazine concentrations in resulting wines without altering fermentation volatiles. Food Res. Int. 2012, 47, 70–79. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G. Application of plastic polymers in remediating wine with elevated alkyl-methoxypyrazine levels. Food Add Contam. Part A 2015, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Pickering, G. Novel Applications for Biomaterials: The Case of Remediation of Wine Taints Using Poly-lactic Acid Polymer. AMM 2015, 749, 70–73. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacruot, M.; Desobry, S. Poly-lactic acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Tauber, V.; Shishoo, R. Influencing of processing parameters on the degradation of poly(l-lactide) during extrusion. J. Appl. Polym. Sci. 2001, 79, 2128–2135. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Savitha, K.; Singhal, R.S.; Kanetkar, V.R. Scalping of Flavors in Packaged Foods. Compr. Food Sci. Food Saf. 2007, 6, 17–35. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B. The Effect of the Timing of Leaf Removal on L. cv. Pinot Noir Berry Ripening, Flavor and Aroma Compounds. Ph.D. Thesis, Lincoln University, Lincoln, Canterbury, New Zealand, 30 September 2010. [Google Scholar]
- Etiévant, P.X. Wine. In Volatile Compounds in Food; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitive determination of of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- San Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and aromatic sensory descriptors (mainly fresh and dried fruit character) of Spanish red wines can be predicted from their aroma-active chemical composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, E. Characterization of Regional Examples of New Zealand Pinot Noir by Means of Sensory and Chemical Analysis. Ph.D. Thesis, Lincoln University, Lincoln, Canterbury, New Zealand, 6 May 2011. [Google Scholar]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotions PTY Ltd.: Adelaide, SA, Australia, 2013. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.


| Treatment | SO2 Resistant Pigments (A.U.) | Total Red Pigments (A.U.) | Wine Color Density (A.U.) | Wine Hue (A.U.) | Red Pigment Coloration (A.U.) | Total Phenolics (A.U.) |
|---|---|---|---|---|---|---|
| Control spiked | 2.89 a,b | 12.37 | 6.16 a,b | 0.83 | 0.27 | 49.53 a,b |
| Polylactic acid 50 | 2.92 b | 11.17 | 6.07 a | 0.83 | 0.30 | 50.73 b |
| Polylactic acid 200 | 2.89 a,b | 11.35 | 6.14 a,b | 0.85 | 0.30 | 50.85 b |
| Polylactic acid 600 | 2.81 a | 10.97 | 6.02 a | 0.86 | 0.30 | 48.63 a |
| Silicone 50 | 2.87 a,b | 13.03 | 6.11 a,b | 0.85 | 0.27 | 50.17 a,b |
| Silicone 200 | 3.08 c | 10.23 | 6.39 c | 0.87 | 0.34 | 50.77 b |
| Silicone 600 | 2.95 b | 11.00 | 6.28 b,c | 0.86 | 0.31 | 50.30 a,b |
| Aroma Compound | Aroma Descriptors | Odor detection Threshold (µg/L) | Purity (%) | CAS No. | Chemical Supplier |
|---|---|---|---|---|---|
| d11 Ethyl hexanoate ISTD | N/A | N/A | 98.7 | 2159-19-5 | CDN Isotopes, Pointe-Claire, Quebec, Canada. |
| Octanal-d16 ISTD | N/A | N/A | 98 | 1219794-66-7 | CDN Isotopes, Pointe-Claire, Quebec, Canada. |
| Ethyl octanoate | Fruity, apricot, pineapple | 580 a | >99 | 106-32-1 | Sigma Aldrich |
| Ethyl hexanoate | Apple, blackberry | 62 ᵈ | 99 | 123-66-0 | Sigma Aldrich |
| Ethyl butanoate | Acid fruit, candy, strawberry | 20 ᵇ | 99 | 105-54-4 | Sigma Aldrich |
| Ethyl isovalerate | Mint, fruit | 3 ᶜ | 98 | 108-64-5 | Sigma Aldrich |
| Ethyl-2-methylbutyrate | Sweet fruit | 18 ᶜ | 99 | 7452-79-1 | Sigma Aldrich |
| Isoamyl acetate | Banana | 30 ᵇ | 97 | 123-92-2 | SAFC, St. Louis, MO, USA |
| 2-Phenylethanol | Roses | 14,000 ᶜ | 99 | 60-12-8 | Sigma Aldrich |
| 1-Hexanol | Herbal, green, grass | 8000 ᵇ | 99.5 | 111-27-3 | Sigma Aldrich |
| Hexanoic acid | Cheese, sweaty | 420 ᶜ | 99.5 | 142-62-1 | Sigma Aldrich |
| Octanoic acid | Rancid, harsh | 500 ᶜ | 99.5 | 124-07-2 | Sigma Aldrich |
| Compound | Retention Time (min) | Target Ion (m/z) | Confirming Ions (m/z) | Calibration Range (µg/L) | Standard Curve (R²) | % Recovery | % CV | LOD (µg/L) | LOQ (µg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl butyrate | 15.5 | 88 | 101, 60 | 0.148–62.20 | 0.9956 | 74 | 4 | 0.670 | 0.148 |
| Ethyl hexanoate | 26.3 | 88 | 115, 60 | 0.417–142.10 | 0.9979 | 98 | 4 | 0.320 | 0.417 |
| Ethyl isovalerate | 18.2 | 88 | 85, 130 | 0.055–12.70 | 0.9918 | 78 | 2 | 0.035 | 0.055 |
| Ethyl octanoate | 42.5 | 88 | 101, 129 | 0.536–100.94 | 0.9935 | 84 | 5 | 0.376 | 0.536 |
| Ethyl-2-methylbutyrate | 18.0 | 57 | 102, 130 | 0.153–5.91 | 0.9825 | 88 | 2 | 0.078 | 0.153 |
| Hexanol | 22.0 | 56 | 55, 84 | 1.249–433.56 | 0.9935 | 82 | 8 | 0.660 | 1.249 |
| Isoamyl acetate | 19.5 | 87 | 43, 73 | 1.402–352.20 | 0.9935 | 77 | 2 | 0.857 | 1.402 |
| Phenylethanol | 50.0 | 91 | 88, 122 | 36.297–5716.10 | 0.9868 | 119 | 13 | 23.323 | 36.297 |
| Hexanoic acid | 15.8 | 60 | 73, 87 | 2.044–365.87 | 0.9933 | 81 | 6 | 1.425 | 2.044 |
| Octanoic acid | 18.2 | 60 | 73, 101 | 0.401–365.87 | 0.9968 | 97 | 3 | 0.319 | 0.401 |
| Standard | Descriptor | Recipe |
|---|---|---|
| 1 | Red fruit | Wine + 3 drops “cherry” + 2 drops “strawberry” |
| 2 | Dark fruit | Wine + 2 drops “fig” + 2 drops “dark cherry” + 1 drop “linden” + 4 drops ”ripe blackberry” |
| 3 | Dried fruit | Wine + 3 drops “fig” + 2 drops “prune” |
| 4 | Green pepper | Wine + 2 drops “green pepper” |
| 5 | Green beans | Wine + 4 tablespoons of green bean brine from a can of President’s Choice® Green Beans |
| 6 | Spice | Wine + 2 drops “black pepper” + 2 drops “clove” + 5 drops “baking spice” + drops “anise” |
| 7 | Herbal | Wine + 1 drop “eucalyptus” + 1 drops “cedar” + 1 drop “green/herbaceous” |
| 8 | Olives | Wine + 4 tablespoons brine form a can of President’s Choice® Green Olives |
| 9 | Dusty/Dirty | Wine + 1 drop “mineral/wet rock” |
| 10 | Leather/earthy | Wine + 2 drops “ leather” + 1 drop “truffles” + 1 drop “mushroom” |
| 11 | Candy/medicinal | Wine + 8 Jolly Rancher candy + 5 Jujubes, blended |
| 12 | Peanuts | Wine + 3 tablespoons raw peanuts, blended |
| 13 | Grassy/green | Wine + 2 drops “green/herbaceous” + 1 drop “unripe” |
| 14 | Brine | Wine + 2 drops “olives” + 1 teaspoon President’s Choice® Soy Sauce |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botezatu, A.; Kemp, B.S.; Pickering, G.J. Chemical and Sensory Evaluation of Silicone and Polylactic Acid-Based Remedial Treatments for Elevated Methoxypyrazine Levels in Wine. Molecules 2016, 21, 1238. https://doi.org/10.3390/molecules21091238
Botezatu A, Kemp BS, Pickering GJ. Chemical and Sensory Evaluation of Silicone and Polylactic Acid-Based Remedial Treatments for Elevated Methoxypyrazine Levels in Wine. Molecules. 2016; 21(9):1238. https://doi.org/10.3390/molecules21091238
Chicago/Turabian StyleBotezatu, Andreea, Belinda S. Kemp, and Gary J. Pickering. 2016. "Chemical and Sensory Evaluation of Silicone and Polylactic Acid-Based Remedial Treatments for Elevated Methoxypyrazine Levels in Wine" Molecules 21, no. 9: 1238. https://doi.org/10.3390/molecules21091238







