Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29
Abstract
:1. Introduction
2. Results
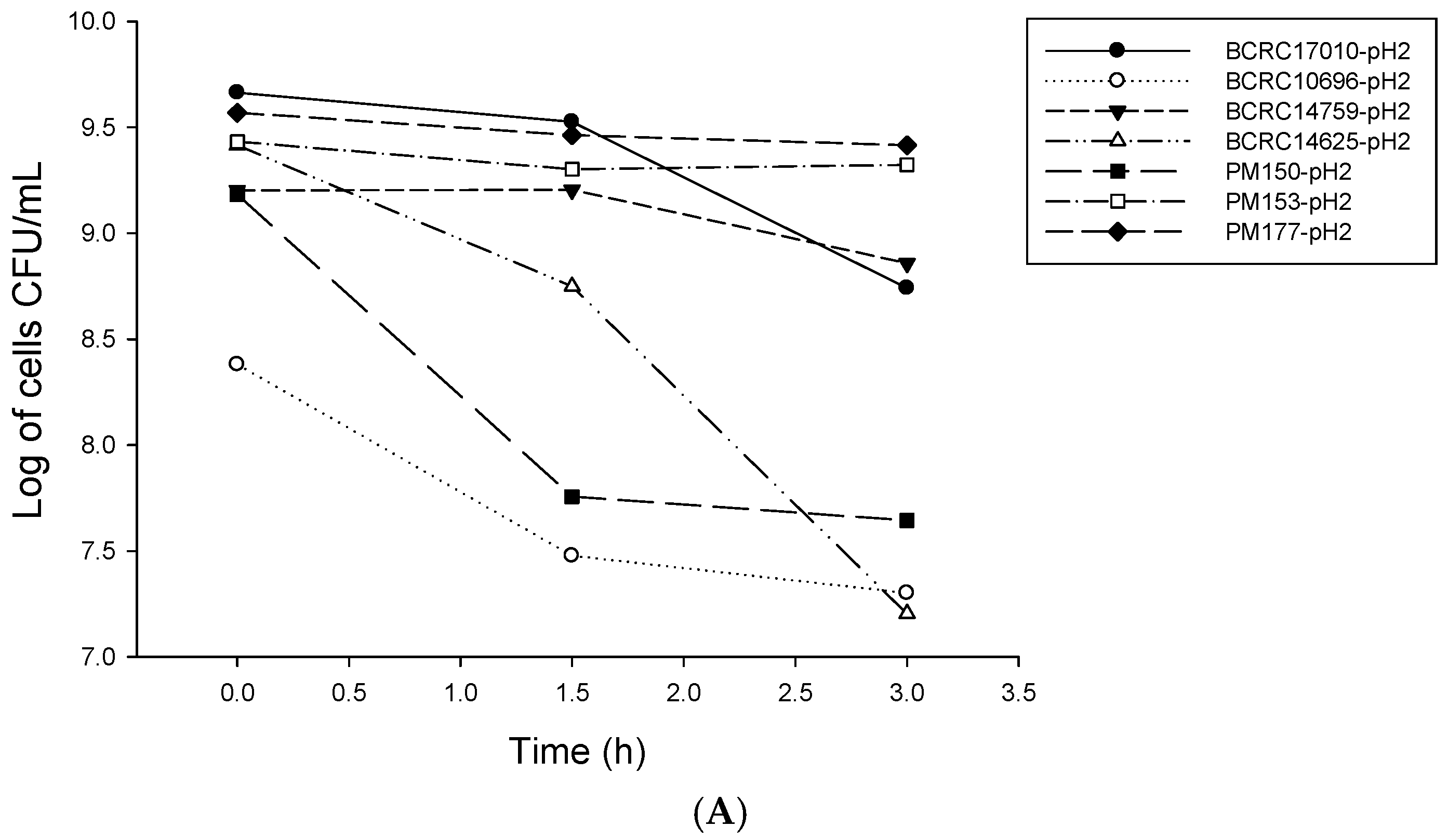
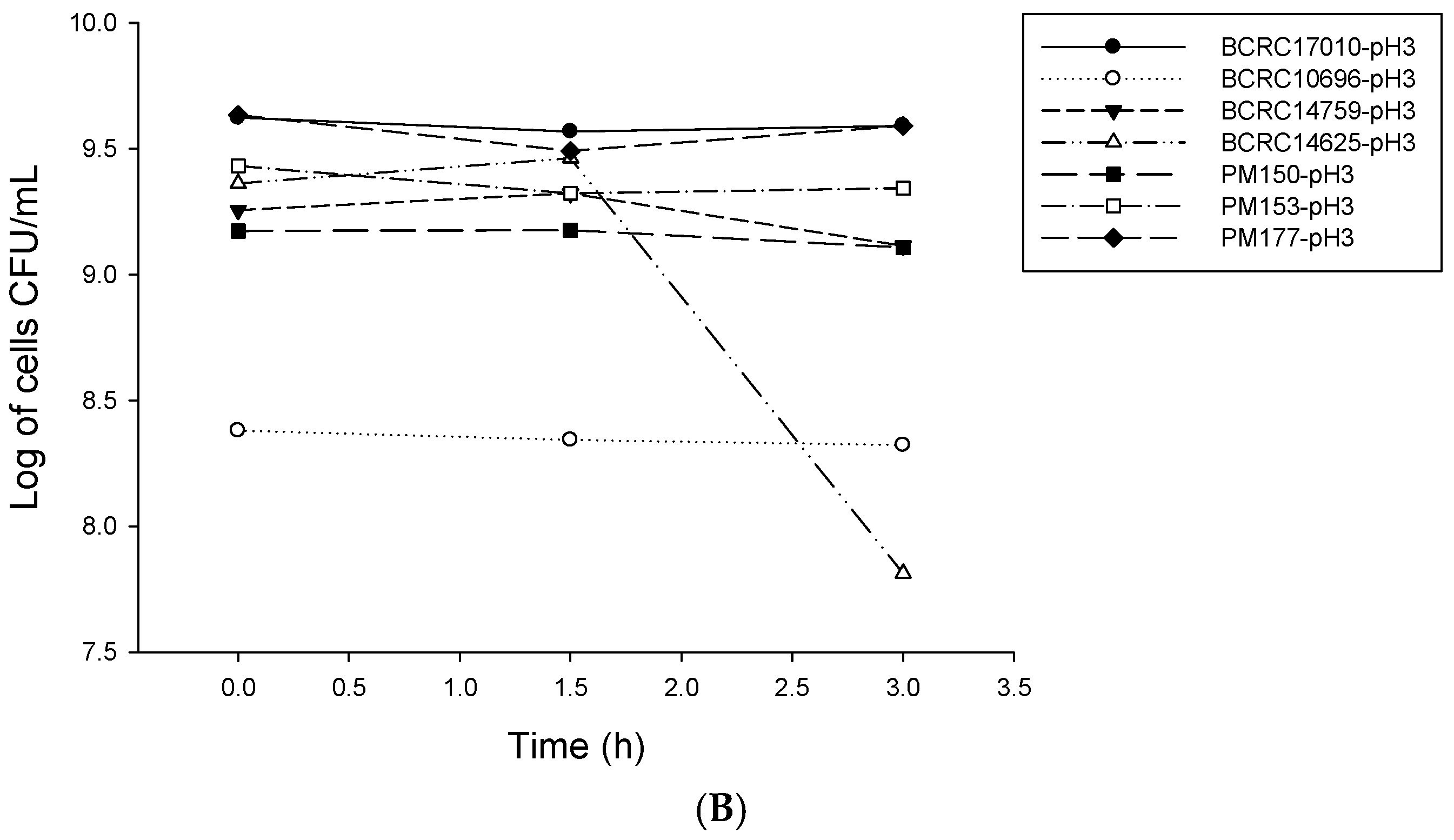
2.1. Analysis of Probiotic Characteristics of Lactobacillus
2.2. Lactobacillus Supernatants Inhibit the Viability of HT-29 Cells
2.3. Damage to HT-29 Cells Caused by Lactobacillus Supernatants and Cells
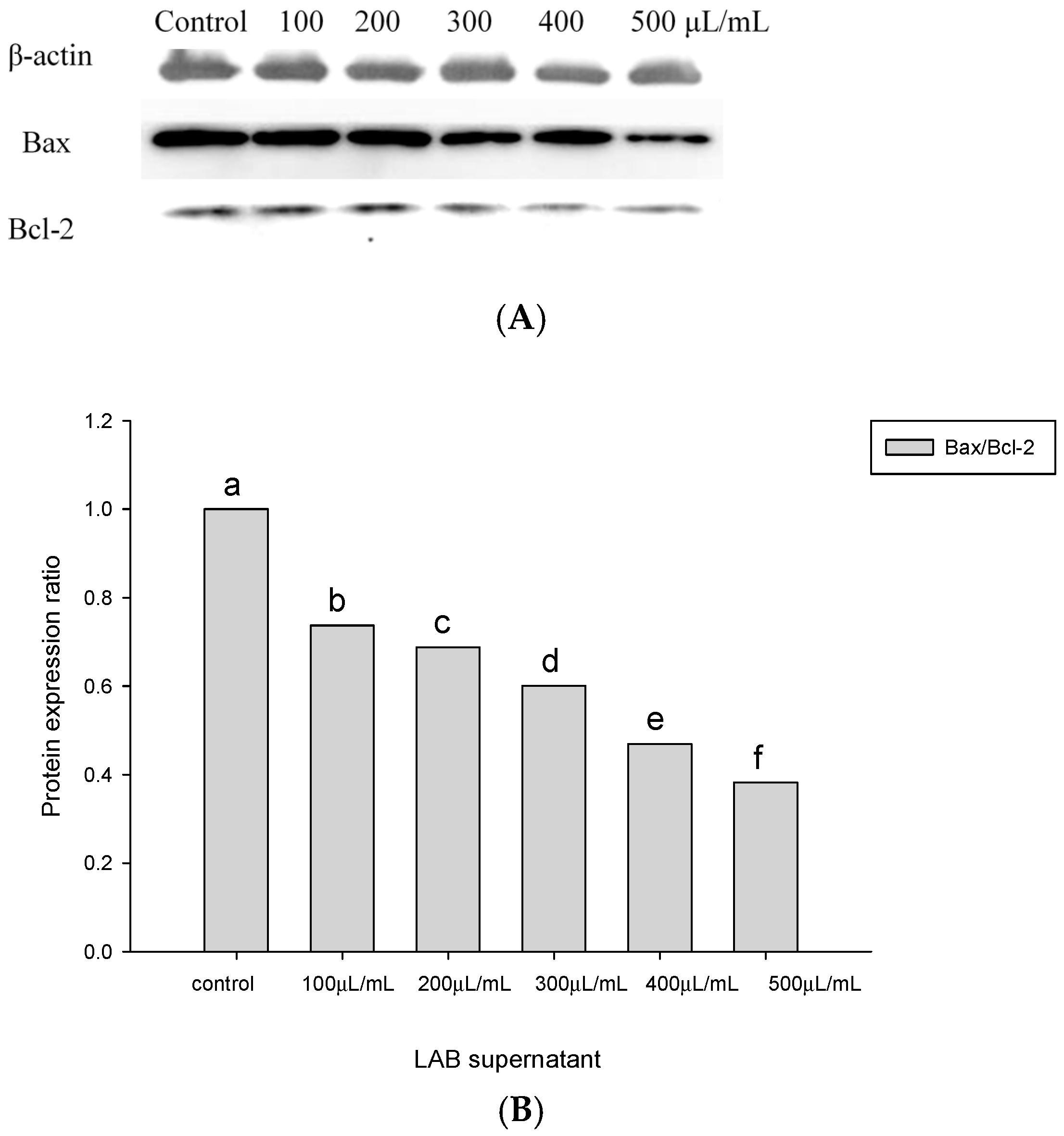
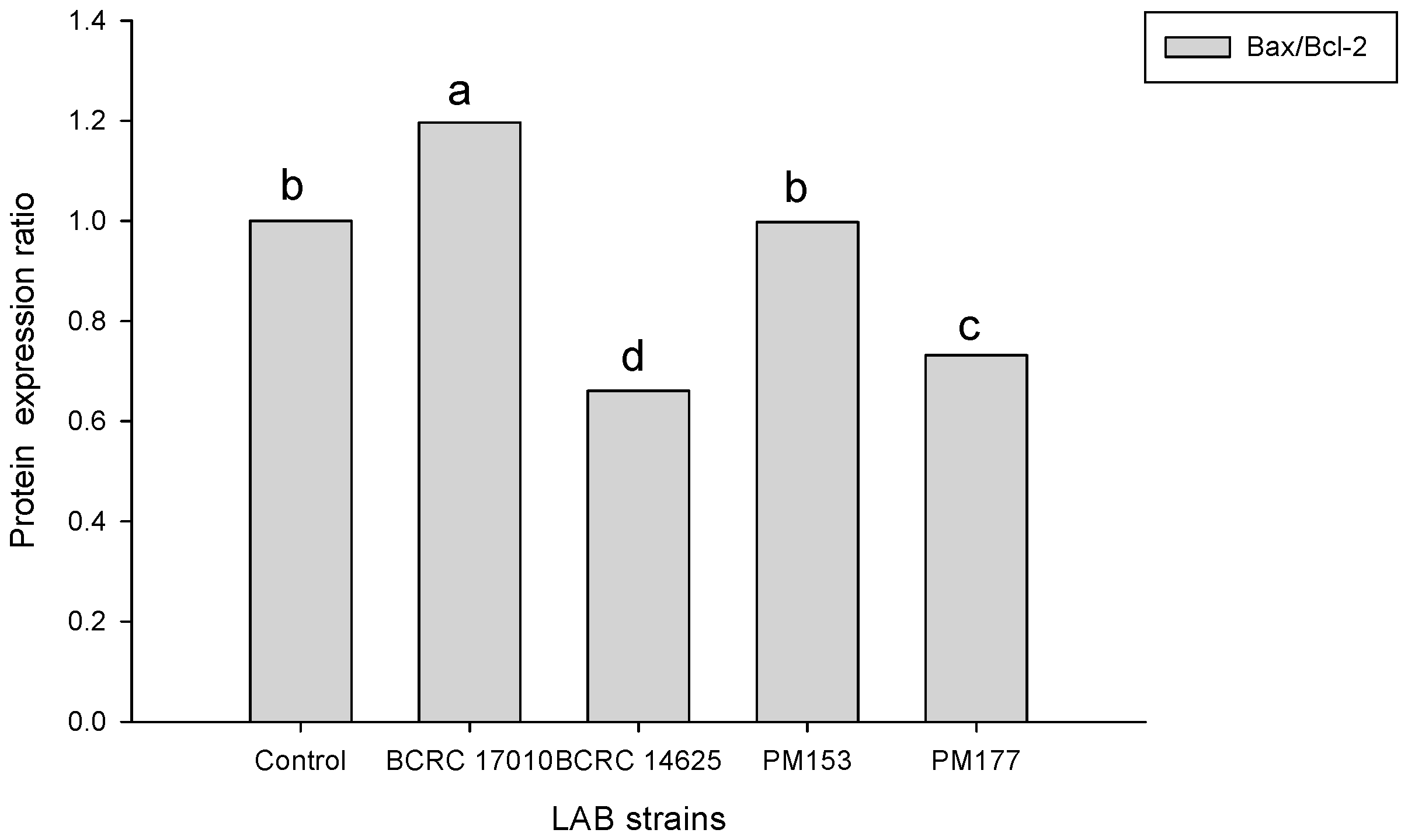
2.4. Apoptosis-Associated Protein Analysis in HT-29 Cells Cultured with Lactobacillus Supernatants and Lactobacillus Cells
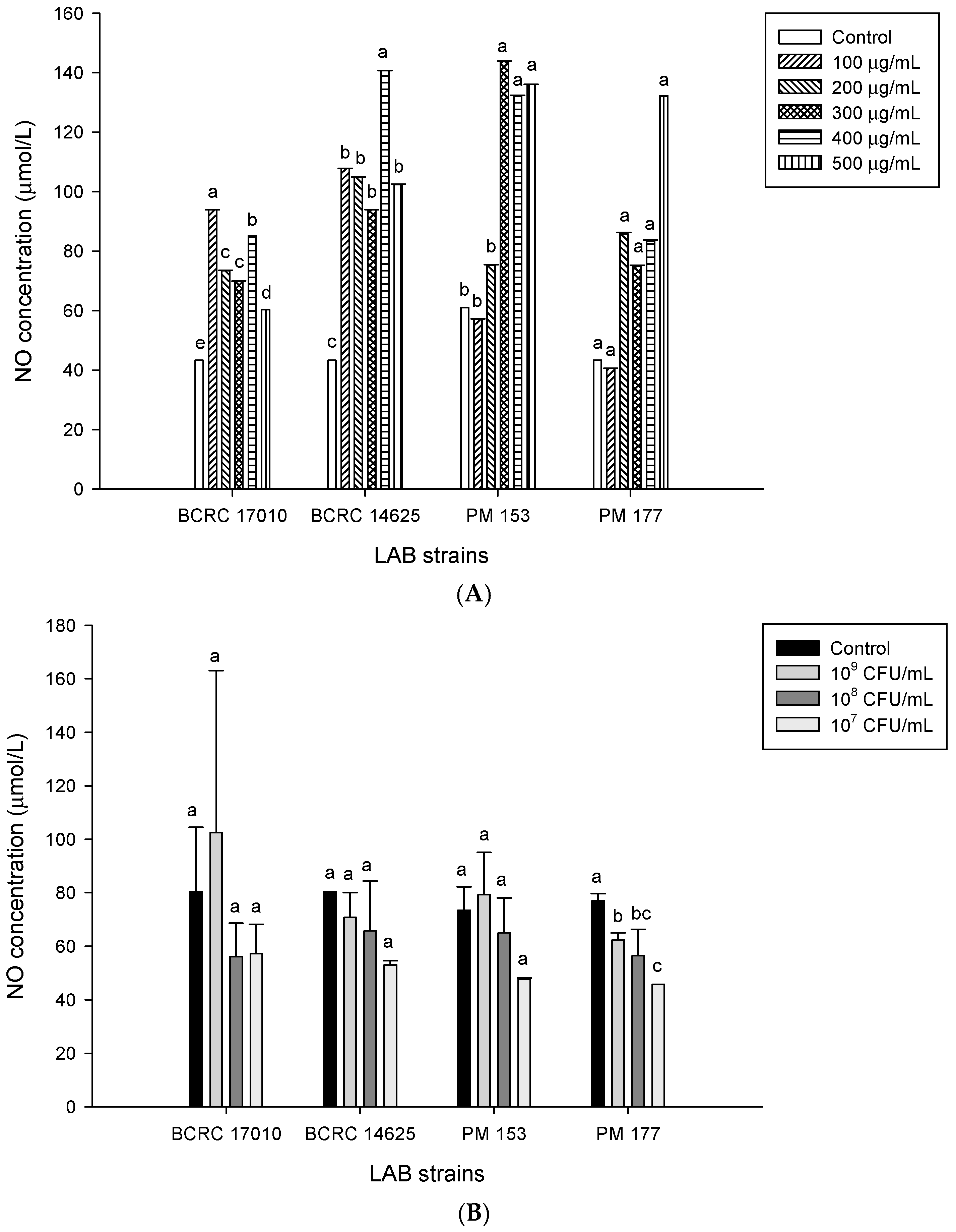
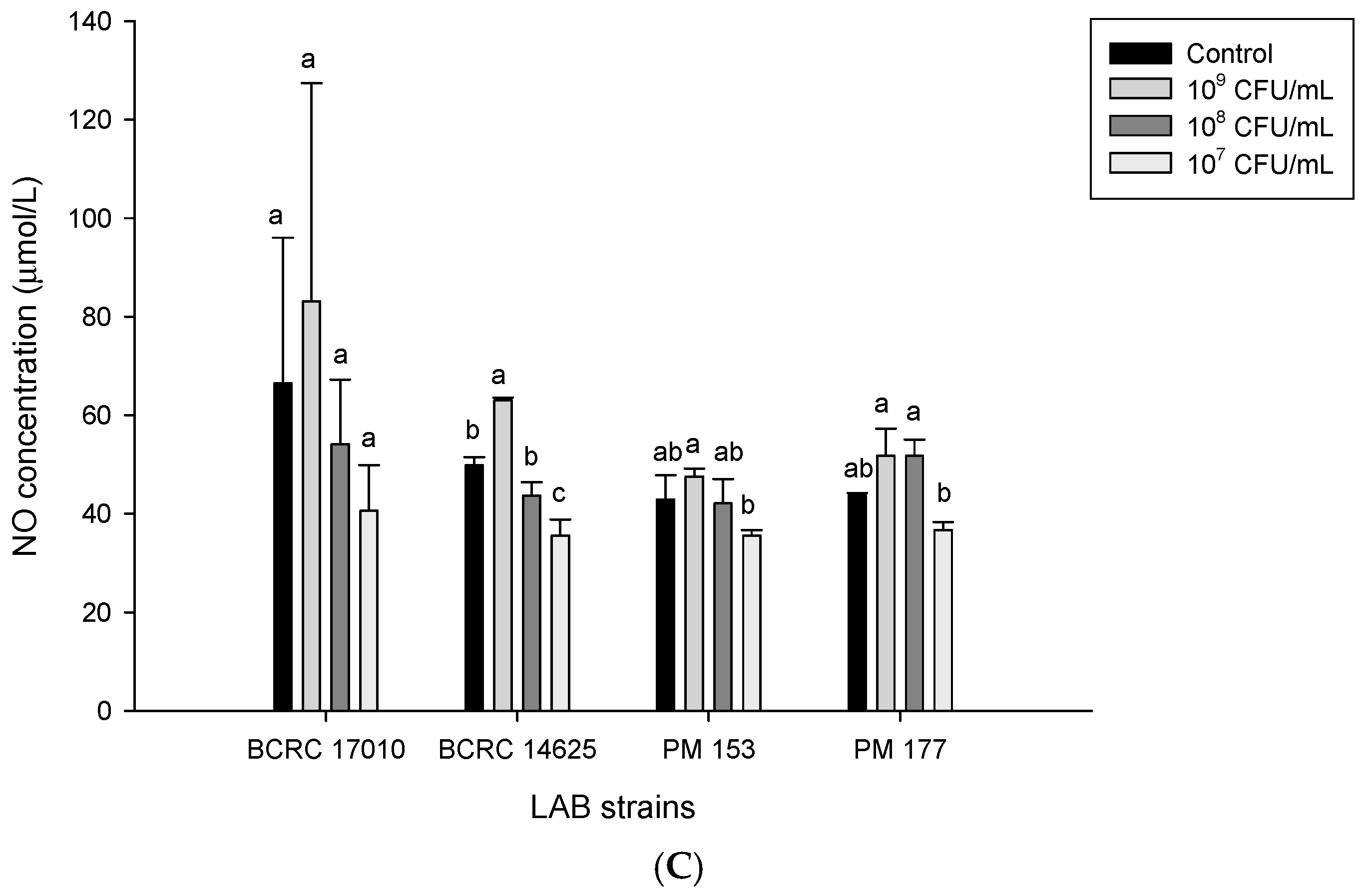
2.5. Capabilities of Lactobacillus Cells and Their Supernatants to Induce NO Production in HT-29 Cells
3. Discussion
4. Materials and Methods
4.1. Bacteria Strains, Culture Medium and Growth Conditions
4.2. LAB Resistance to Simulated Gastrointestinal Conditions
4.3. The Epithelial Cell Line Culture and Adhesion Assay
4.4. Analysis of Cell Viability
4.5. Quantitative Measurements of Bax and Bcl-2 Proteins Involved in the Apoptotic Pathway of Colorectal Cancer Cells
4.6. Analysis of Lactate Dehydrogenase (LDH)
4.7. Analysis of Nitric Oxide (NO) Concentration
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Willett, W.C. Diet, nutrition, and avoidable cancer. Environ. Health. Perspect. 1995, 103, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, P. Review article: The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations; World Health Organization. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agricultural Organization of the United Nations; World Health Organization: Washington, DC, USA, 2002. [Google Scholar]
- Havenaar, R.; Ten Brink, B.; Huis in’t Veld, J.H.J. Selection of strains for Probiotic use. In Probiotics. The Scientific Basis; Fuller, R., Ed.; Chapman & Hall: London, UK, 1992; pp. 209–221. [Google Scholar]
- Vankerckhoven, V.; Huys, G.; Vancanneyt, M.; Snauwaert, C.; Swings, J.; Klare, I.; Witte, W.; van Autgaerden, T.; Chapelle, S.; Lammens, C.; et al. Genotypic diversity, antimicrobial resistance, and virulence factors of human isolates and probiotic cultures constituting two intraspecific groups of Enterococcus faecium isolates. Appl. Environ. Microbiol. 2008, 74, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Langerholc, T. Functional cell models of the gut and their applications in food microbiology—A review. Int. J. Food Microbiol. 2010, 141, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Servin, A.L. Pathogenesis of human enterovirulent bacteria: Lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol. Mol. Biol. Rev. 2013, 77, 380–439. [Google Scholar] [CrossRef] [PubMed]
- Baricault, L.; Denariaz, G.; Houri, J.J.; Bouley, C.; Sapin, C.; Trugnan, G. Use of HT-29, a cultured human colon cancer cell line, to study the effect of fermented milks on colon cancer cell growth and differentiation. Carcinogenesis 1995, 16, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nakatsugi, S.; Watanabe, K.; Kawamori, T.; Ishikawa, F.; Morotomi, M.; Sugie, S.; Toda, T.; Sugimura, T.; Wakabayashi, K. Inhibitory effects of bifidobacterium-fermented soy milk on 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine-induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones. Carcinogenesis 2000, 21, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, I.A.; el-Sayed, E.M.; Hafez, S.A.; el-Zeini, H.M.; Saleh, F.A. Inhibitory effect of yoghurt and soya yoghurt containing bifidobacteria on the proliferation of Ehrlich ascites tumor cells in vitro and in vivo in a mouse tumor model. Br. J. Nutr. 2004, 92, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Commanea, D.; Hughes, R.; Shortt, C.; Rowland, I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res. 2005, 591, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, K.; Rafer, J. The role of probiotic bacteria in cancer prevention. Microbes Infect 2000, 2, 681–686. [Google Scholar] [CrossRef]
- Ningegowda, M.A.; Gurudutt, P.S. In vitro fermentation of prebiotics by Lactobacillus plantarum CFR 2194: Selectivity, viability and effect of metabolites on β-glucuronidase activity. World J. Microb. Biot. 2012, 28, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Muganga, L.; Liu, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Screening for lactic acid bacteria based on antihyperglycaemic and probiotic potential and application in synbiotic set yoghurt. J. Funct. Foods 2015, 16, 125–136. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, P.; Dimova, D.; Pichereau, V.; Auffray, Y.; Boyaval, P.; Jan, G. Susceptibility and adaptive response to bile salts in propionibacterium freudenreichii: Physiological and proteomic analysis. Appl. Environ. Microbiol. 2003, 69, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Molenaar, D.; de Vos, W.M.; Kleerebezem, M. DNA micro-array-based identification of bile-responsive genes in Lactobacillus plantarum. J. Appl. Microbiol. 2006, 100, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Hsieh, H.Y.; King, A.E.; Chi, L.L.; Tsena, J.H. To pre-challenge lactic acid bacteria with simulated gastrointestinal conditions is a suitable approach to studying potential probiotic properties. J. Microbiol. Methods 2014, 107, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Lagadic-Gossmann, D.; Lemaire, C.; Brenner, C.; Jan, G. Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate, major end-products of the human probiotic propionibacteria. Apoptosis 2007, 12, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Shirzad, M.; Akrami, S.M.; Mousavi, A.S.; Mirsalehian, A.; Modarressi, M.H. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013, 62, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Mohammadi, F.; Fazeli, H.; Mirlohi, H. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran. J. Basic Med. Sci. 2014, 17, 815–819. [Google Scholar] [PubMed]
- Wang, S.; Zhang, L.; Fan, R.; Han, X.; Yi, H.; Zhang, L.; Xue, C.; Li, H.; Zhang, Y.; Shigwedha, N. Induction of HT-29 cells apoptosis by lactobacilli isolated from fermented products. Res. Microbiol. 2014, 165, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, W.; Xiao, W.; Liang, H.; Yang, Y.; Yang, H. Angiotensin II induces apoptosis in intestinal epithelial cells through the AT2 receptor, GATA-6 and the Bax pathway. Biochem. Biophys. Res. Commun. 2012, 424, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.; El-Hayek, S.; Tarras, O.; El-Sabban, M.; El-Sibai, M.; Rizk, S. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int. J. Oncol. 2014, 45, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Iyer, C.; Kosters, A.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B.; Versalovic, J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell. Microbiol. 2008, 10, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Sobko, T.; Huang, L.; Midtvedt, T.; Norin, E.; Gustafsson, L.E.; Norman, M.; Jansson, E.Å.; Lundberg, J.O. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006, 41, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Messmer, U.K.; Brune, B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem. J. 1995, 319, 299–305. [Google Scholar] [CrossRef]
- Fern’andaz, M.F.; Boris, S.; Barbes, C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 2003, 94, 449–455. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, A.; Shi, Z.; He, C.; Ding, J.; Wang, X.; Ma, J.; Zhang, H. A mitochondria-mediated apoptotic pathway induced by deoxynivalenol in human colon cancer cells. Toxicol. In Vitro 2012, 26, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| LAB Strains | pH of Supernatant | l-Lactic Acid Content (mM) |
|---|---|---|
| PM150 | 3.85 ± 0.19 b | 144.87 ± 7.06 bc |
| PM153 | 3.73 ± 0.04 b | 189.83 ± 0.00 a |
| PM177 | 3.81 ± 0.11 b | 174.84 ± 21.19 ab |
| BCRC10696 | 4.09 ± 0.09 ab | 139.88 ± 0.00 bcd |
| BCRC14625 | 4.25 ± 0.07 a | 104.91 ± 21.09 cd |
| BCRC14759 | 4.03 ± 0.37 ab | 99.91 ± 28.26 d |
| BCRC17010 | 3.77 ± 0.07 b | 199.82 ± 14.13 a |
| Inhibition Ratio (%) | |||||
|---|---|---|---|---|---|
| l-Lactic Acid Levels | 10 mM | 50 mM | 100 mM | 150 mM | 200 mM |
| pH 4.5 | 58 a | 113 b | 155 c | 155 c | 155 c |
| pH 5.5 | 60 a | 45 a | 103 b | 109 b | 149 c |
| pH 6.5 | 73 a | 95 ab | 71 a | 97 ab | 116 c |
| pH 7.5 | 60 a | 45 b | 81 c | 93 d | 116 e |
| Concentration | Inhibition Ratio (%) | IC50 | ||||||
|---|---|---|---|---|---|---|---|---|
| Strains | 200 μL/mL | 300 μL/mL | 400 μL/mL | 500 μL/mL | 600 μL/mL | 700 μL/mL | ||
| BCRC17010 | −39.2 | −6.5 | 45.2 | 73.0 | 75.6 | 99.9 | 479.2 | |
| BCRC10696 | −81.9 | −84.2 | −31.7 | 44.1 | 63.5 | 58.5 | 609.8 | |
| BCRC14625 | 25.7 | 39.8 | 55.1 | 71.0 | 78.4 | 99.9 | 370.7 | |
| BCRC14759 | 4.5 | 24.5 | 43.8 | 62.3 | 63.3 | 43.5 | 467.9 | |
| PM150 | −227.6 | −176.9 | −94.9 | −42.7 | 23.6 | 52.6 | 667.5 | |
| PM153 | 27.1 | 50.6 | 75.0 | 86.5 | 89.4 | 100.0 | 299.3 | |
| PM177 | 56.6 | 62.0 | 74.4 | 84.3 | 88.4 | 99.9 | 134.9 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-Y.; Hsieh, Y.-M.; Huang, C.-C.; Tsai, C.-C. Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules 2017, 22, 107. https://doi.org/10.3390/molecules22010107
Chen Z-Y, Hsieh Y-M, Huang C-C, Tsai C-C. Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules. 2017; 22(1):107. https://doi.org/10.3390/molecules22010107
Chicago/Turabian StyleChen, Zhung-Yuan, You-Miin Hsieh, Chun-Chih Huang, and Cheng-Chih Tsai. 2017. "Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29" Molecules 22, no. 1: 107. https://doi.org/10.3390/molecules22010107
APA StyleChen, Z.-Y., Hsieh, Y.-M., Huang, C.-C., & Tsai, C.-C. (2017). Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules, 22(1), 107. https://doi.org/10.3390/molecules22010107





