Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro
Abstract
:1. Introduction
2. Results
2.1. Component Analysis of R. isatidis Polysaccharides
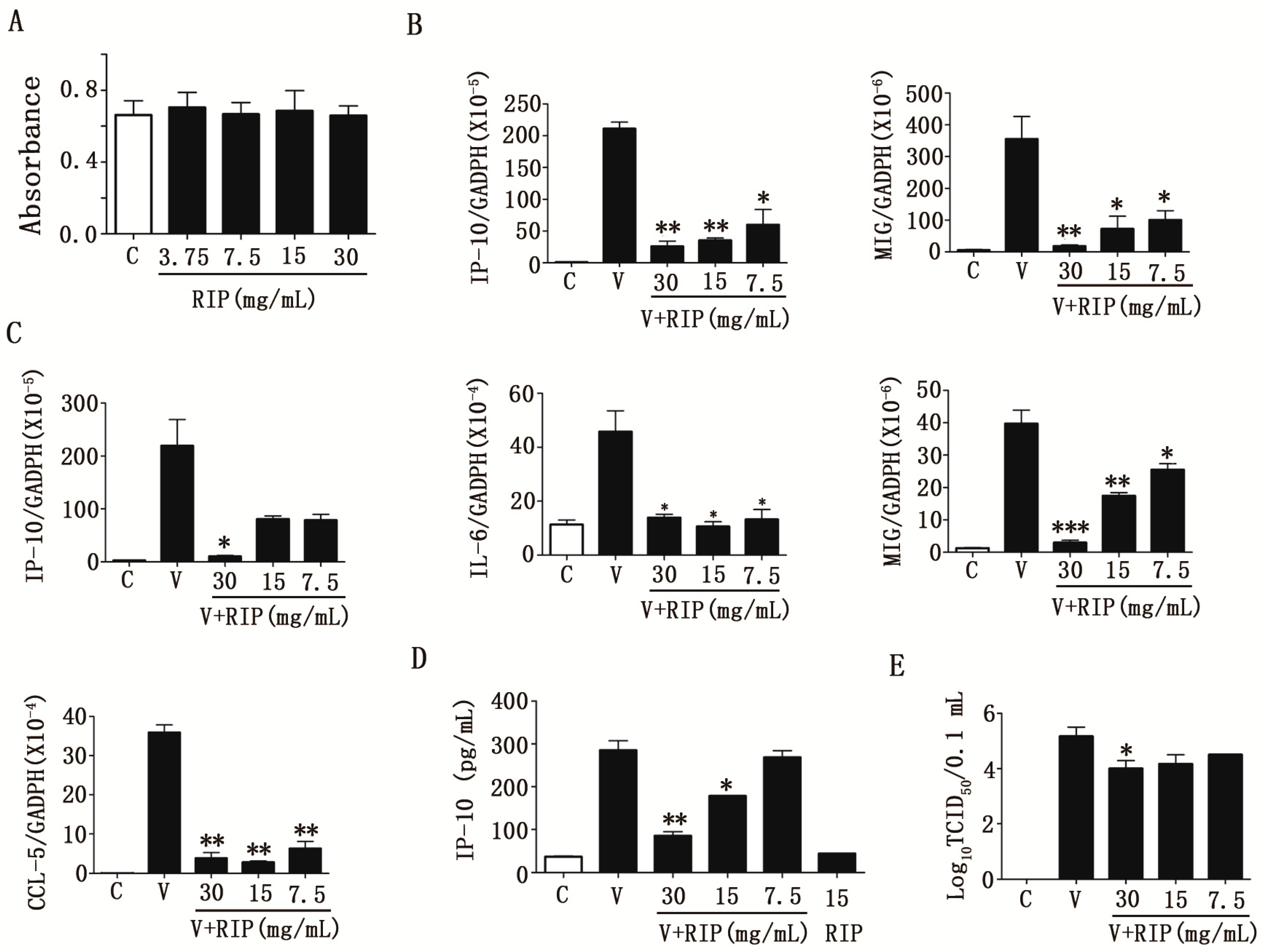
2.2. Anti-Influenza a Virus Activities of R. isatidis Polysaccharides
2.3. R. isatidis Polysaccharides Can Effectively Inhibit Human Influenza Virus PR8/H1N1-Induced Cytokine Expression in 16HBE Cells
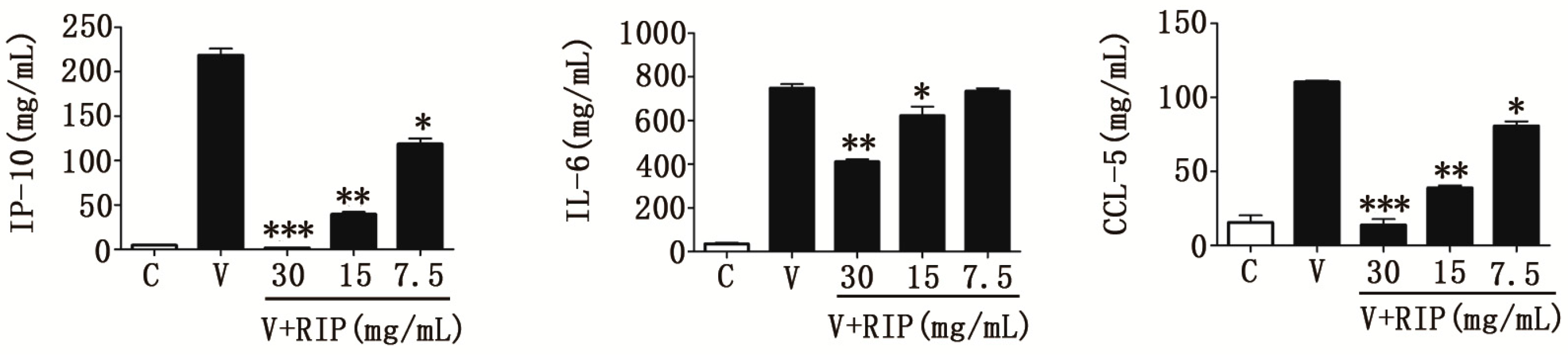
2.4. R. isatidis Polysaccharides Can Inhibit Pro-Inflammatory Cytokine Expression Induced by H9N2 Subtype Avian Influenza a Viruses
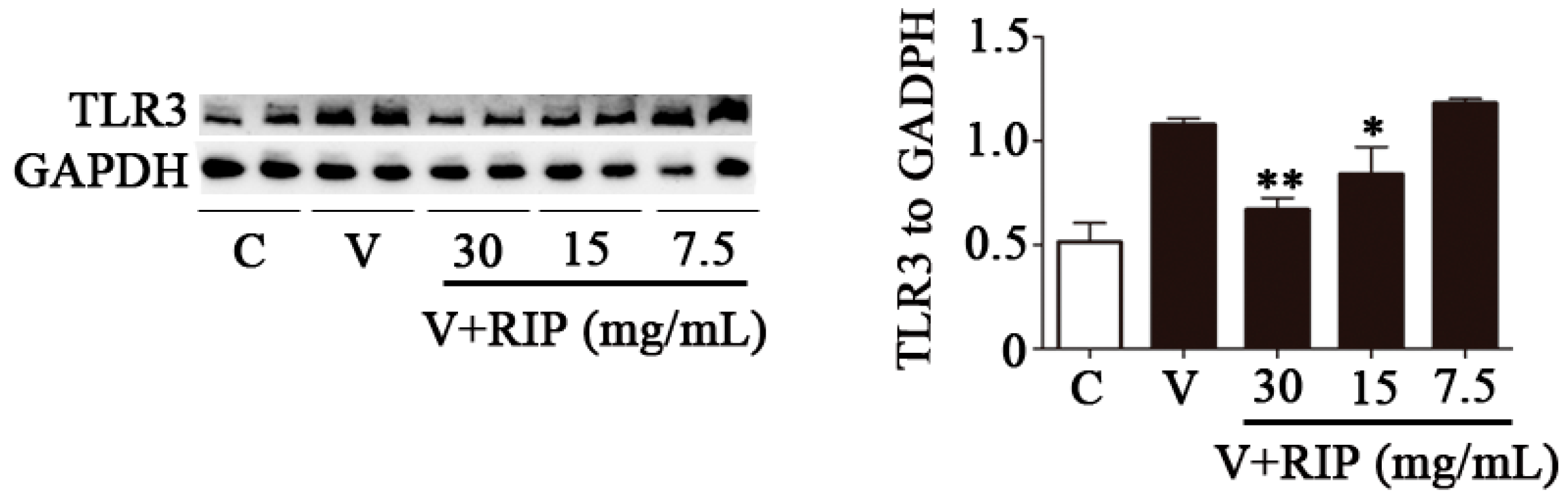
2.5. R. isatidis Polysaccharides Can Inhibit the Protein Expression Level of TLR3 Induced by PR8/H1N1 Virus in 16HBE Cells
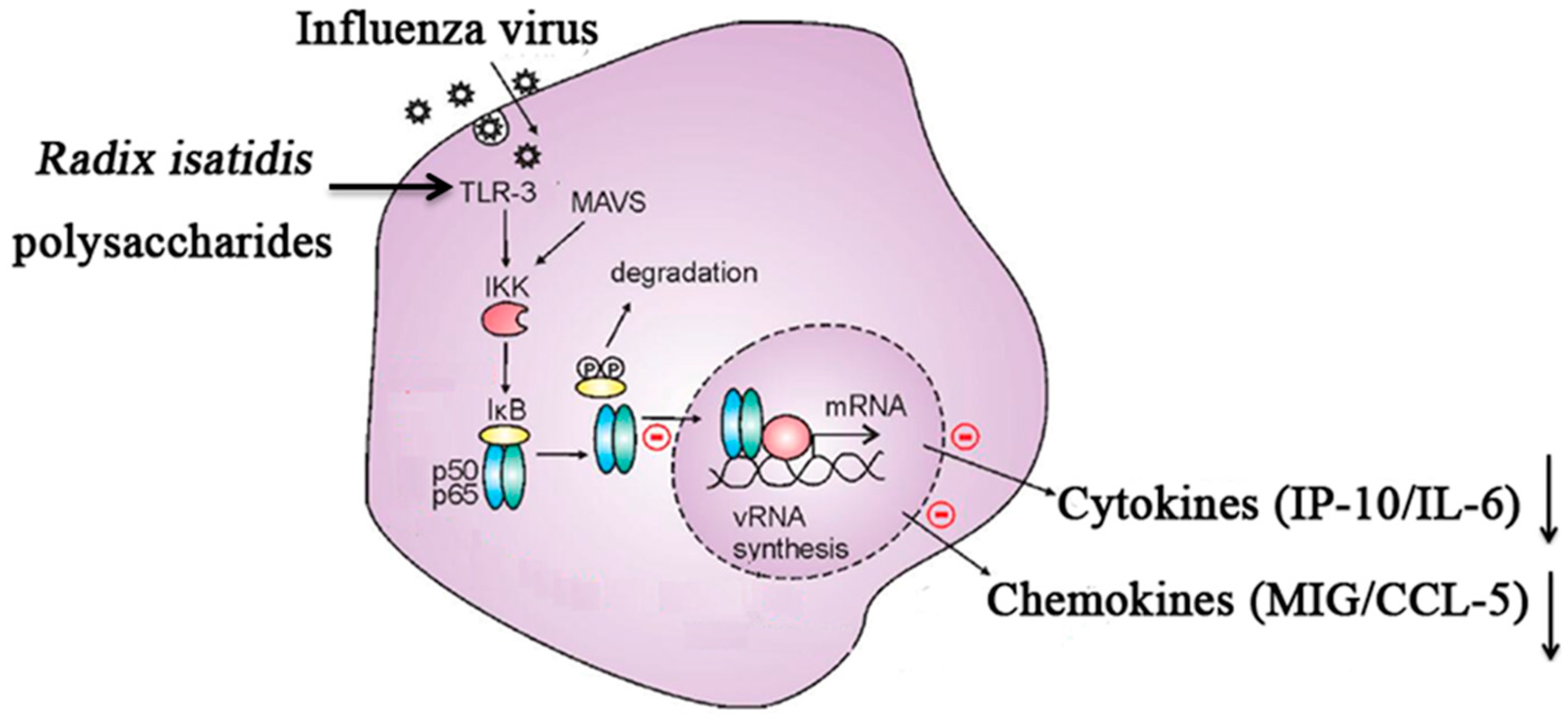
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of the Polysaccharide
4.2. Cells and Viruses
4.3. Cytotoxicity Assay
4.4. Cytopathic Effect (CPE) Inhibition Assay
4.5. Cell Treatment and Inflammatory Cytokine Analysis
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IAV | Influenza A virus |
| RIP | Radix isatidis polysaccharides |
| TLR-3 | Toll-like receptor-3 |
| CPE | Cytopathic effect |
| SI | Selectivity index |
References
- Dienz, O.; Rud, J.G.; Eaton, S.M.; Lanthier, P.A.; Burg, E.; Drew, A.; Bunn, J.; Suratt, B.T.; Haynes, L.; Rincon, M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012, 5, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wan, J.; Qian, K.; Liu, X.; Xiao, Z.; Sun, J.; Zeng, Z.; Wang, Q.; Zhang, J.; Jiang, G.; et al. Clinical characteristics of human infection with a novel avian-origin influenza a(H10N8) virus. Chin. Med. J. (Engl.) 2014, 127, 3238–3242. [Google Scholar] [PubMed]
- Yang, Z.F.; Mok, C.K.; Peiris, J.S.; Zhong, N.S. Human infection with a novel avian influenza a(H5N6) virus. N. Engl. J. Med. 2015, 373, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Mok, C.K.; Liu, X.Q.; Li, X.B.; He, J.F.; Guan, W.D.; Xu, Y.H.; Pan, W.Q.; Chen, L.Y.; Lin, Y.P.; et al. Clinical, virological and immunological features from patients infected with re-emergent avian-origin human H7N9 influenza disease of varying severity in guangdong province. PLoS ONE 2015, 10, e0117846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampson, A.W. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann. Acad. Med. Singap. 2008, 37, 510–517. [Google Scholar] [PubMed]
- De Clercq, E. Antivirals and antiviral strategies. Nat. Rev. Microbiol 2004, 2, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Infectious diseases: Novel agents fight flu infection. Nat. Rev. Drug Discov. 2013, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, M.; Zhao, Y.; Ling, F.; Xiao, K.; Li, Q.; Li, B.; Lu, C.; Qi, W.; Zeng, Z.; et al. Saikosaponin a inhibits influenza a virus replication and lung immunopathology. Oncotarget 2015, 6, 42541–42556. [Google Scholar] [PubMed]
- Zhou, J.; Wang, D.; Gao, R.; Zhao, B.; Song, J.; Qi, X.; Zhang, Y.; Shi, Y.; Yang, L.; Zhu, W.; et al. Biological features of novel avian influenza a (H7N9) virus. Nature 2013, 499, 500–503. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza a (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; Steinmueller, M.; von Wulffen, W.; Cakarova, L.; Pinto, R.; Pleschka, S.; Mack, M.; Kuziel, W.A.; Corazza, N.; Brunner, T.; et al. Lung epithelial apoptosis in influenza virus pneumonia: The role of macrophage-expressed tnf-related apoptosis-inducing ligand. J. Exp. Med. 2008, 205, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Baillie, J.K.; Digard, P. Influenza—Time to target the host? N. Engl. J. Med. 2013, 369, 191–193. [Google Scholar] [PubMed]
- Ramos, I.; Fernandez-Sesma, A. Modulating the innate immune response to influenza a virus: Potential therapeutic use of anti-inflammatory drugs. Front. Immunol. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zheng, Z.; Zhao, S.; Zhao, J.; Lin, Q.; Li, C.; Zhu, Q.; Zhong, N. Antiviral activity of isatis indigotica root-derived clemastanin b against human and avian influenza a and b viruses in vitro. Int. J. Mol. Med. 2013, 31, 867–873. [Google Scholar] [PubMed]
- Zuo, L.; Li, J.B.; Xu, J.; Yang, J.Z.; Zhang, D.M.; Tong, Y.L. Studies on chemical constituents in root of isatis indigotica. China J. Chin. Mater. Med. 2007, 32, 688–691. [Google Scholar]
- Li, L.; Qiao, C.Z.; Li, X.L.; Dong, T.Y. Study of quality control on herbal drugs and preparations of daqingye and banlangen. Yao Xue Xue Bao 1993, 28, 229–233. [Google Scholar] [PubMed]
- Mak, N.K.; Leung, C.Y.; Wei, X.Y.; Shen, X.L.; Wong, R.N.; Leung, K.N.; Fung, M.C. Inhibition of rantes expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem. Pharmacol. 2004, 67, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.; Kang, S.S.; Chan, R.W.; Yue, P.Y.; Mak, N.K.; Poon, L.L.; Wong, R.N.; Peiris, J.S.; Chan, M.C. Anti-inflammatory and antiviral effects of indirubin derivatives in influenza a (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells. Antivir. Res. 2014, 106, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B.; Li, C.; Chen, Q.; Wang, Y.; Li, Z.; Chen, T.; Yang, C.; Jiang, Z.; Zhong, N.; et al. Lariciresinol-4-O-beta-d-glucopyranoside from the root of isatis indigotica inhibits influenza a virus-induced pro-inflammatory response. J. Ethnopharmacol. 2015, 174, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.K.; Kim, D.H.; Lim, H.; Shin, H.K.; Kim, J.K. The anti-inflammatory effects of a methanolic extract from Radix isatidis in murine macrophages and mice. Inflammation 2010, 33, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zhong, S.; Zhao, S.; Zeng, X.; Mo, Z.; Qin, S.; Guan, W.; Li, C.; Zhong, N. In vitro inhibition of influenza virus infection by a crude extract from isatis indigotica root resulting in the prevention of viral attachment. Mol. Med. Rep. 2012, 5, 793–799. [Google Scholar] [PubMed]
- Guo, H.X.-L.; Wang, Y.; Yang, Z.; Wang, Y.; Li, Z.; Hu, P. System isolation and purification of Radix isatidis polysaccharides and determination of their compositions. Chin. Tradit. Herb. Drugs 2016, 47, 1508–1514. [Google Scholar]
- Liu, H.; Zhang, W.; Dong, S.; Song, L.; Zhao, S.; Wu, C.; Wang, X.; Liu, F.; Xie, J.; Wang, J.; et al. Protective effects of sea buckthorn polysaccharide extracts against lps/d-galn-induced acute liver failure in mice via suppressing tlr4-nf-kappab signaling. J. Ethnopharmacol. 2015, 176, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Wang, Y.T.; Qin, S.; Zhao, S.S.; Zhao, Y.S.; Lin, Q.; Guan, W.D.; Huang, Q.D.; Mo, Z.Y.; Li, C.Y.; et al. The effects of a hot water soluble extract (S-03) isolated from isatis indigotica root on inf luenza a and b viruses in vitro. Chin. J. Virol. 2011, 27, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Okumura, Y.; Yamada, H.; Le, T.Q.; Yano, M. Proteases essential for human influenza virus entry into cells and their inhibitors as potential therapeutic agents. Curr. Pharm. Des. 2007, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, H.; Zhang, Z.; Li, P. Antioxidant and anti-inflammatory activities of Radix isatidis polysaccharide in murine alveolar macrophages. Int. J. Biol. Macromol. 2013, 58, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Walsh, K.B.; Rice, S.; Rosen, H.; Oldstone, M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Yeung, A.C.; Chan, P.K. Apoptosis, cytokine and chemokine induction by non-structural 1 (NS1) proteins encoded by different influenza subtypes. Virol. J. 2011, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhu, Y.; Wen, T.; Cui, L.; Ge, Y.; Jiao, Y.; Wu, T.; Ge, A.; Ji, H.; Xu, K.; et al. Cytokine and chemokine levels in patients infected with the novel avian influenza a (H7N9) virus in china. J. Infect. Dis. 2013, 208, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Wong, C.K.; Chan, P.K.; Chan, M.C.; Wong, R.Y.; Lun, S.W.; Ngai, K.L.; Lui, G.C.; Wong, B.C.; Lee, S.K.; et al. Cytokine response patterns in severe pandemic 2009 h1n1 and seasonal influenza among hospitalized adults. PLoS ONE 2011, 6, e26050. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Balloy, V.; Lagranderie, M.; Alexopoulou, L.; Escriou, N.; Flavell, R.; Chignard, M.; Si-Tahar, M. Detrimental contribution of the toll-like receptor (TLR)3 to influenza a virus-induced acute pneumonia. PLoS Pathog. 2006, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Miller, D.J.; Bowman, E.R.; Nagarkar, D.R.; Schneider, D.; Zhao, Y.; Linn, M.J.; Goldsmith, A.M.; Bentley, J.K.; Sajjan, U.S.; et al. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 2011, 7, e1002070. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.K.; Pillai, P.S.; Iwasaki, A. Efficient influenza a virus replication in the respiratory tract requires signals from tlr7 and rig-i. Proc. Natl. Acad. Sci. USA 2013, 110, 13910–13915. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zhong, Y.; Zhang, Y.; Liu, J.P.; Wang, Y.F.; Jia, W.N.; Wang, G.C.; Li, Z.; Zhu, Y.; Gao, X.M. A network analysis of the chinese medicine lianhua-qingwen formula to identify its main effective components. Mol. Biosyst. 2016, 12, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Muench, L.J.R.A.H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Sample Availability: Samples of the compounds extrate from Radix isatidis are available from the authors.
| Virus Type and Strain | Polysaccharide | Oseltamivir | ||||
|---|---|---|---|---|---|---|
| TC50 (mg/mL) | IC50 (mg/mL) | SI | TC5 (mg/mL) | IC50 (mg/mL) | SI | |
| A/PR/8/34 (H1N1) | >40 | 20.48 ± 0.31 | >1.95 | >0.312 | 0.000238 ± 0.000015 | >1000 |
| A/Guangzhou/GIRD07/09 (H1N1) | >40 | 8.47 ± 0.07 | >4.72 | >0.312 | 0.000194 ± 0.000009 | >1000 |
| A/Aichi/2/68 (H3N2) | >40 | 4.35 ± 0.05 | >9.20 | >0.312 | 0.00138 ± 0.00017 | >100 |
| A/Duck/Guangdong (H6N2) | >40 | 28.20 ± 0.49 | >1.42 | >0.312 | 0.00537 ± 0.00019 | >50 |
| A/Chicken/Guangdong/1996 (H9N2) | >40 | 20.57 ± 0.25 | >1.94 | >0.312 | 0.00466 ± 0.00010 | >50 |
| Gene | Primers and Probe | Sequence (5′→3′) |
|---|---|---|
| IL-6 | Forward | 5′-CGGGAACGAAAGAGAAGCTCTA-3′ |
| Reverse | 5′-CGCTTGTGGAGAAGGAGTTCA-3′ | |
| Probe | 5′-FAM-TCCCCTCCAGGAGCCCAGCT-3′TAMRA | |
| IP-10 | Forward | 5′-GAAATTATTCCTGCAAGCCAATTT-3′ |
| Reverse | 5′-TCACCCTTCTTTTTCAT-TGTAGCA-3′ | |
| Probe | 5′-FAM-TCCACGTGTTGAGATCA-3′MGB | |
| MIG | Forward | 5′-TCTTGCTGGTTCTGATTGGAGTG-3′ |
| Reverse | 5′-GATAGTCCCTTGGTTGGTGCTG-3′ | |
| Probe | 5′-FAM-CAGGAACAGCGACCCTTTCTCACTACTGG-3′BHQ-1 | |
| CCL-5 | Forward | 5′-CAGCAGTCGTCTTTGTCACC-3′ |
| Reverse | 5′-GTTGATGTACTCCCGAACCC-3′ | |
| Probe | 5′-FAM-CGCCAAGTGTGTGCCAACCC-3′TAMRA | |
| GAPDH | Forward | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| Reverse | 5′-GAAGATGGTGATGGGATTTC-3′ | |
| Probe | 5′-FAM-CAAGCTTCCCGTTCTCAGCC-3′TAMRA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules 2017, 22, 116. https://doi.org/10.3390/molecules22010116
Li Z, Li L, Zhou H, Zeng L, Chen T, Chen Q, Zhou B, Wang Y, Chen Q, Hu P, et al. Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules. 2017; 22(1):116. https://doi.org/10.3390/molecules22010116
Chicago/Turabian StyleLi, Zhengtu, Li Li, Hongxia Zhou, Lijuan Zeng, Tingting Chen, Qiaolian Chen, Beixian Zhou, Yutao Wang, Qiaoyan Chen, Ping Hu, and et al. 2017. "Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro" Molecules 22, no. 1: 116. https://doi.org/10.3390/molecules22010116
APA StyleLi, Z., Li, L., Zhou, H., Zeng, L., Chen, T., Chen, Q., Zhou, B., Wang, Y., Chen, Q., Hu, P., & Yang, Z. (2017). Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules, 22(1), 116. https://doi.org/10.3390/molecules22010116






