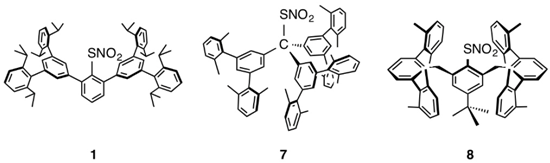
Modeling of the Bioactivation of an Organic Nitrate by a Thiol to Form a Thionitrate Intermediate
Abstract
:1. Introduction
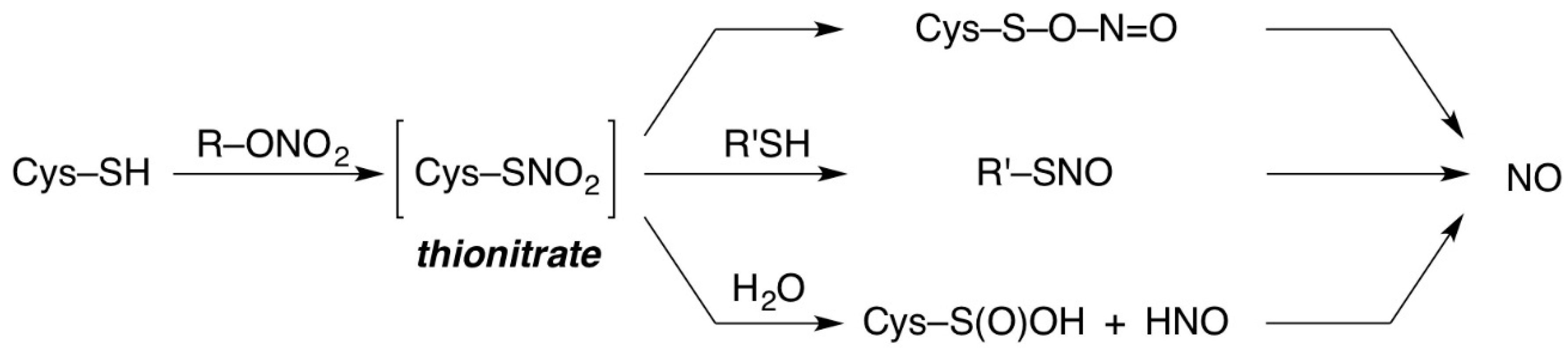
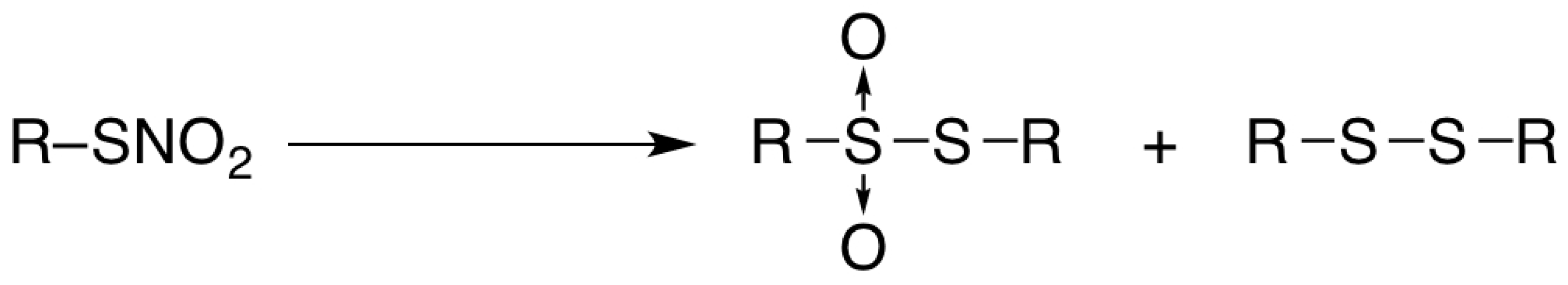
2. Results and Discussion
3. Materials and Methods
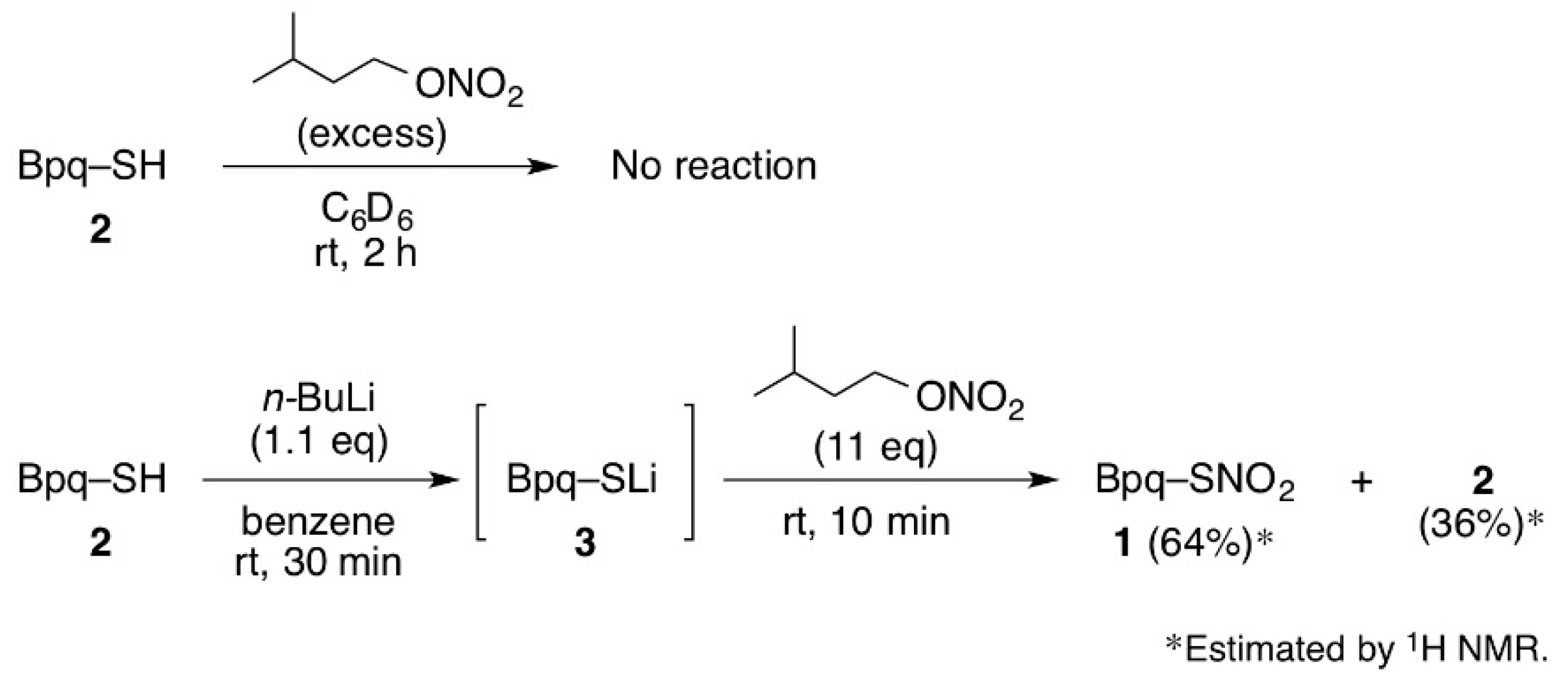
3.1. The Reaction of Thiol 2 with Isoamyl Nitrate
3.2. Synthesis of Thionitrate 1
3.3. Synthesis of Thionitrate 6
3.4. Formation of 6 by the Reaction with Isosorbide-5-Mononitrate (ISMN)
3.5. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fung, H.L. Biochemical mechanism of nitroglycerin action and tolerance: Is this old mystery solved? Ann. Rev. Pharmacol. Toxicol. 2004, 44, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Stamler, J.S. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc. Med. 2006, 16, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Beretta, M. The enigma of nitroglycerin bioactivation and nitrate tolerance: News, views and troubles. Br. J. Pharmacol. 2008, 155, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Münzel, T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid. Redox Signaling 2015, 23, 899–942. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.G.J.; Weldon, H. NO problem for nitroglycerin: Organic nitrate chemistry and therapy. Chem. Soc. Rev. 1998, 27, 331–337. [Google Scholar] [CrossRef]
- Wenzl, M.V.; Beretta, M.; Griesberger, M.; Russwurm, M.; Koesling, D.; Schmidt, K.; Mayer, B.; Gorren, A.C.F. Site-directed mutagenesis of aldehyde dehydrogenase-2 suggests three distinct pathways of nitroglycerin biotransformation. Mol. Pharmacol. 2011, 80, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Yeates, R.A.; Laufen, H.; Leitold, M. The reaction between organic nitrates and sulfhydryl compounds. A possible model system for the activation of organic nitrates. Mol. Pharmacol. 1985, 28, 555–559. [Google Scholar] [PubMed]
- Yeates, R.A. Possible mechanism of activation of soluble guanylate-cyclase by organic nitrates. Drug Res. 1992, 42, 1314–1317. [Google Scholar]
- Lang, B.S.; Gorren, A.C.F.; Oberdorfer, G.; Wenzl, M.V.; Furdui, C.M.; Poole, L.B.; Mayer, B.; Gruber, K. Vascular bioactivation of nitroglycerin by aldehyde dehydrogenase-2. J. Biol. Chem. 2012, 287, 38124–38134. [Google Scholar] [CrossRef] [PubMed]
- Oae, S.; Shinhama, K. Organic thionitrites and related substances—A review. Org. Prep. Proced. Int. 1983, 15, 165–198. [Google Scholar] [CrossRef]
- Oae, S.; Shinhama, K.; Fujimori, K.; Kim, Y.H. Physical-properties and various reactions of thionitrites and related substances. Bull. Chem. Soc. Jpn. 1980, 53, 775–784. [Google Scholar] [CrossRef]
- Artz, J.D.; Yang, K.X.; Lock, J.; Sanchez, C.; Bennett, B.M.; Thatcher, G.R.J. Reactivity of thionitrate esters: Putative intermediates in nitrovasodilator activity. Chem. Commun. 1996, 927–928. [Google Scholar] [CrossRef]
- Goto, K.; Shimada, K.; Furukawa, S.; Miyasaka, S.; Takahashi, Y.; Kawashima, T. Formation of a stable sulfenic acid by hydrolysis of a thionitrate and a sulfenyl bromide. Chem. Lett. 2006, 35, 862–863. [Google Scholar] [CrossRef]
- Goto, K.; Yoshikawa, S.; Ideue, T.; Sase, S. Transnitrosation from a stable thionitrate to an amine with concomitant formation of a sulfenic acid. J. Sulfur Chem. 2013, 34, 705–710. [Google Scholar] [CrossRef]
- Goto, K. Synergy of reactivity and stability in nanoscale molecular architectures. In Synergy in Supramolecular Chemistry; Nabeshima, T., Ed.; CRC Press: Boca Raton, FA, USA, 2015; pp. 191–218. [Google Scholar]
- Goto, K.; Hino, Y.; Kawashima, T.; Kaminaga, M.; Yano, E.; Yamamoto, G.; Takagi, N.; Nagase, S. Synthesis and crystal structure of a stable S-nitrosothiol bearing a novel steric protection group and of the corresponding S-nitrothiol. Tetrahedron Lett. 2000, 41, 8479–8483. [Google Scholar] [CrossRef]
- Goto, K.; Hino, Y.; Takahashi, Y.; Kawashima, T.; Yamamoto, G.; Takagi, N.; Nagase, S. Synthesis, structure, and reactions of the first stable aromatic S-nitrosothiol bearing a novel dendrimer-type steric protection group. Chem. Lett. 2001, 30, 1204–1205. [Google Scholar] [CrossRef]
- Ishihara, M.; Abe, N.; Sase, S.; Goto, K. Synthesis, structure, and reactivities of a stable primary-alkyl-substituted sulfenic acid. Chem. Lett. 2015, 44, 615–617. [Google Scholar] [CrossRef]
- Sase, S.; Aoki, Y.; Abe, N.; Goto, K. Stable sulfenyl iodide bearing a primary alkyl steric protection group with a cavity-shaped framework. Chem. Lett. 2009, 38, 1188–1189. [Google Scholar] [CrossRef]
- Sase, S.; Kakimoto, R.; Goto, K. Synthesis of a stable selenoaldehyde by self-catalyzed thermal dehydration of a primary-alkyl-substituted selenenic acid. Angew. Chem. Int. Ed. 2015, 54, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Sase, S.; Kakimoto, R.; Kimura, R.; Goto, K. Synthesis of a stable primary-alkyl-substituted selenenyl iodide and its hydrolytic conversion to the corresponding selenenic acid. Molecules 2015, 20, 21415–21420. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Sonoda, D.; Shimada, K.; Sase, S.; Kawashima, T. Modeling of the 5′-deiodination of thyroxine by iodothyronine deiodinase: Chemical corroboration of a selenenyl iodide intermediate. Angew. Chem. Int. Ed. 2010, 49, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Takenaka, K.; Okazaki, R.; Takeda, N.; Tokitoh, N. The first stable aromatic S-nitrosothiol: Synthesis, structure and reactivity. Chem. Lett. 2001, 30, 1206–1207. [Google Scholar] [CrossRef]
- Itoh, M.; Takenaka, K.; Okazaki, R. Synthesis, structure, and reactivity of stable aromatic S-nitrothiols bearing bulky substituents. Nippon Joshi Daigaku Kiyo Rigakubu 2004, 12, 33–38. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Computer Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Not available.
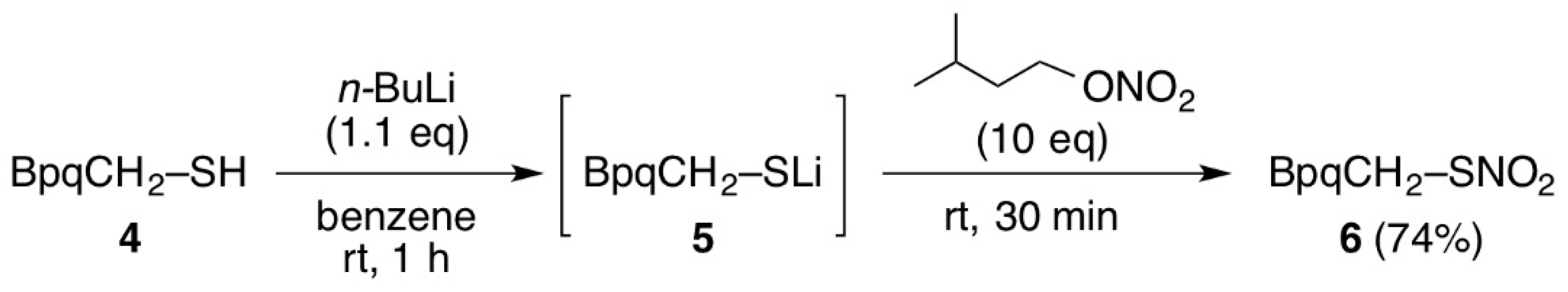

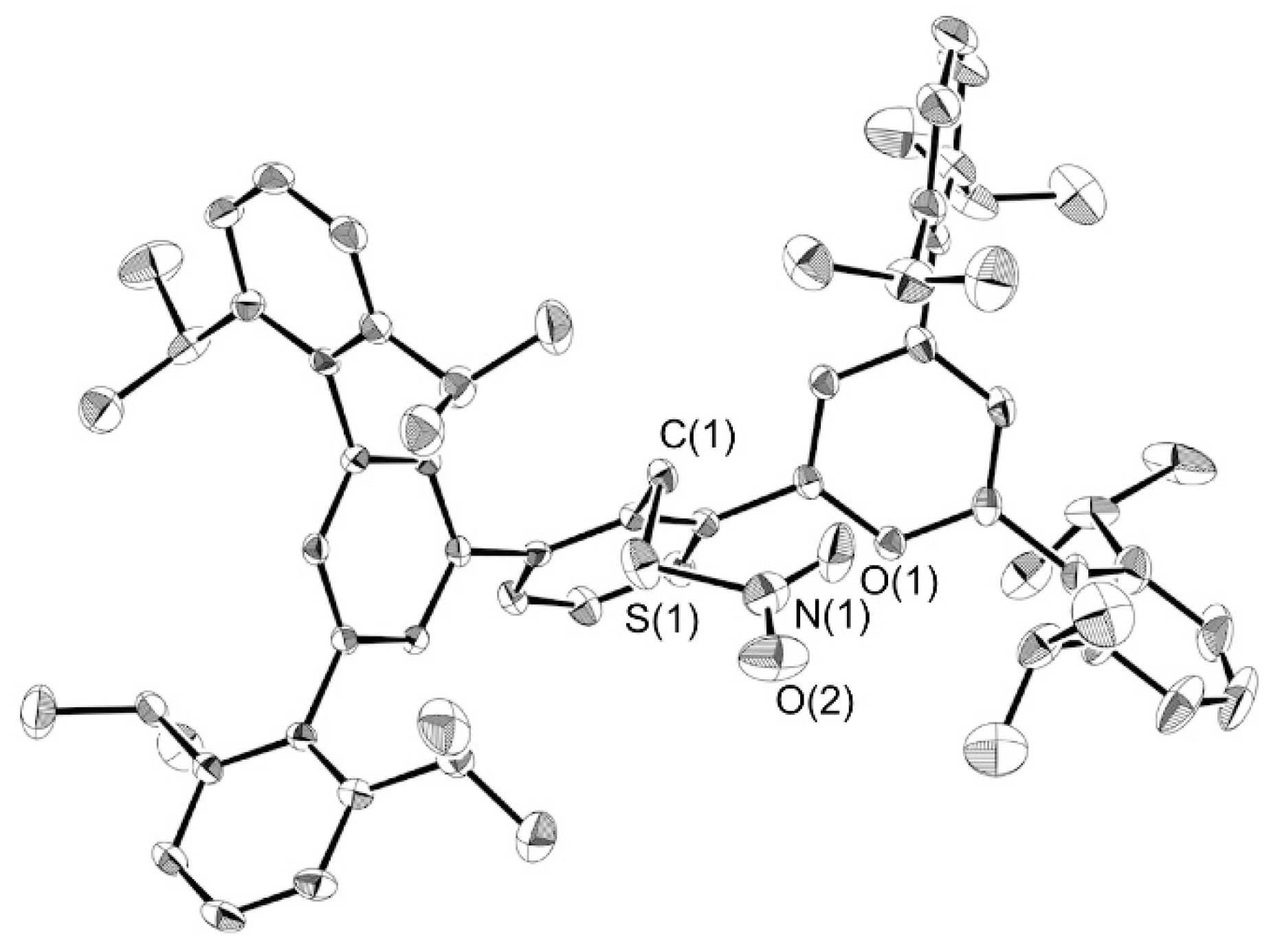
| 6 (this work) | 1 [14] | 7 [16] | 8 [24] | |
| Bond lengths (Å) | ||||
| S(1)–N(1) | 1.780 (6) | 1.7898 (17) | 1.746 (9) | 1.795 (2) |
| N(1)–O(1) | 1.205 (8) | 1.2180 (19) | 1.229 (9) | 1.215 (3) |
| N(1)–O(2) | 1.211 (6) | 1.213 (2) | 1.239 (9) | 1.228 (3) |
| C(1)–S(1) | 1.803 (3) | 1.7651 (15) | 1.789 (6) | 1.764 (2) |
| Bond angles (°) | ||||
| S(1)–N(1)–O(1) | 121.7 (4) | 120.25 (12) | 114.1 (9) | 121.19 (17) |
| S(1)–N(1)–O(2) | 114.1 (9) | 113.28 (13) | 119.7 (9) | 113.48 (18) |
| O(1)–N(1)–O(2) | 124.00 (57) | 126.44 (17) | 126.2 (11) | 125.3 (2) |
| C(1)–S(1)–N(1) | 100.5 (2) | 100.47 (7) | 107.9 (5) | 99.75 (10) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, T.; Shimada, K.; Aoki, Y.; Kawashima, T.; Sase, S.; Goto, K. Modeling of the Bioactivation of an Organic Nitrate by a Thiol to Form a Thionitrate Intermediate. Molecules 2017, 22, 19. https://doi.org/10.3390/molecules22010019
Sano T, Shimada K, Aoki Y, Kawashima T, Sase S, Goto K. Modeling of the Bioactivation of an Organic Nitrate by a Thiol to Form a Thionitrate Intermediate. Molecules. 2017; 22(1):19. https://doi.org/10.3390/molecules22010019
Chicago/Turabian StyleSano, Tsukasa, Keiichi Shimada, Yohei Aoki, Takayuki Kawashima, Shohei Sase, and Kei Goto. 2017. "Modeling of the Bioactivation of an Organic Nitrate by a Thiol to Form a Thionitrate Intermediate" Molecules 22, no. 1: 19. https://doi.org/10.3390/molecules22010019