Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model
Abstract
:1. Introduction
2. Results and Discussion
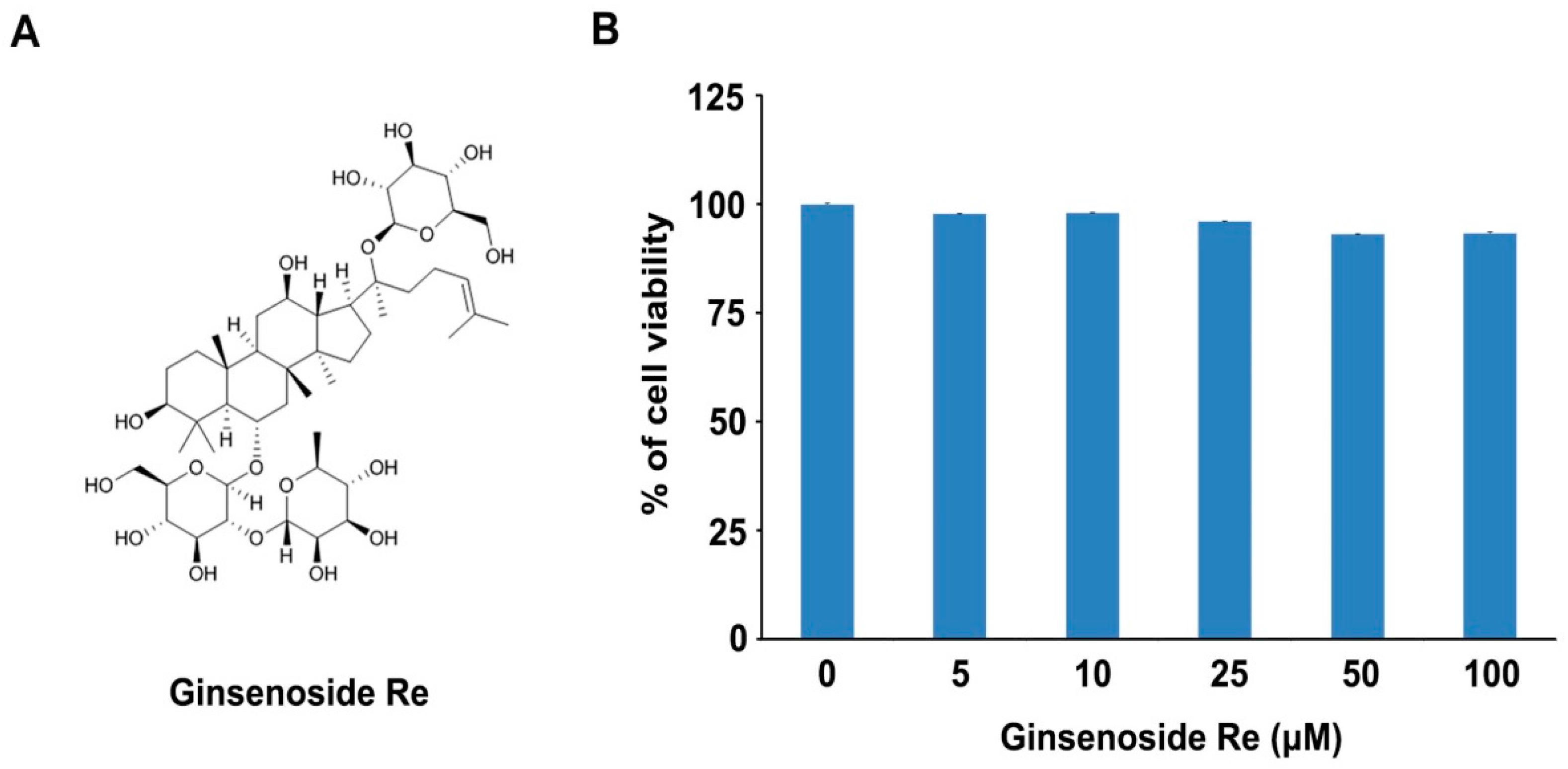
2.1. Effects of Ginsenoside Re on Cell Viability in Mouse Osteoblast Precursor MC3T3-E1 Cells
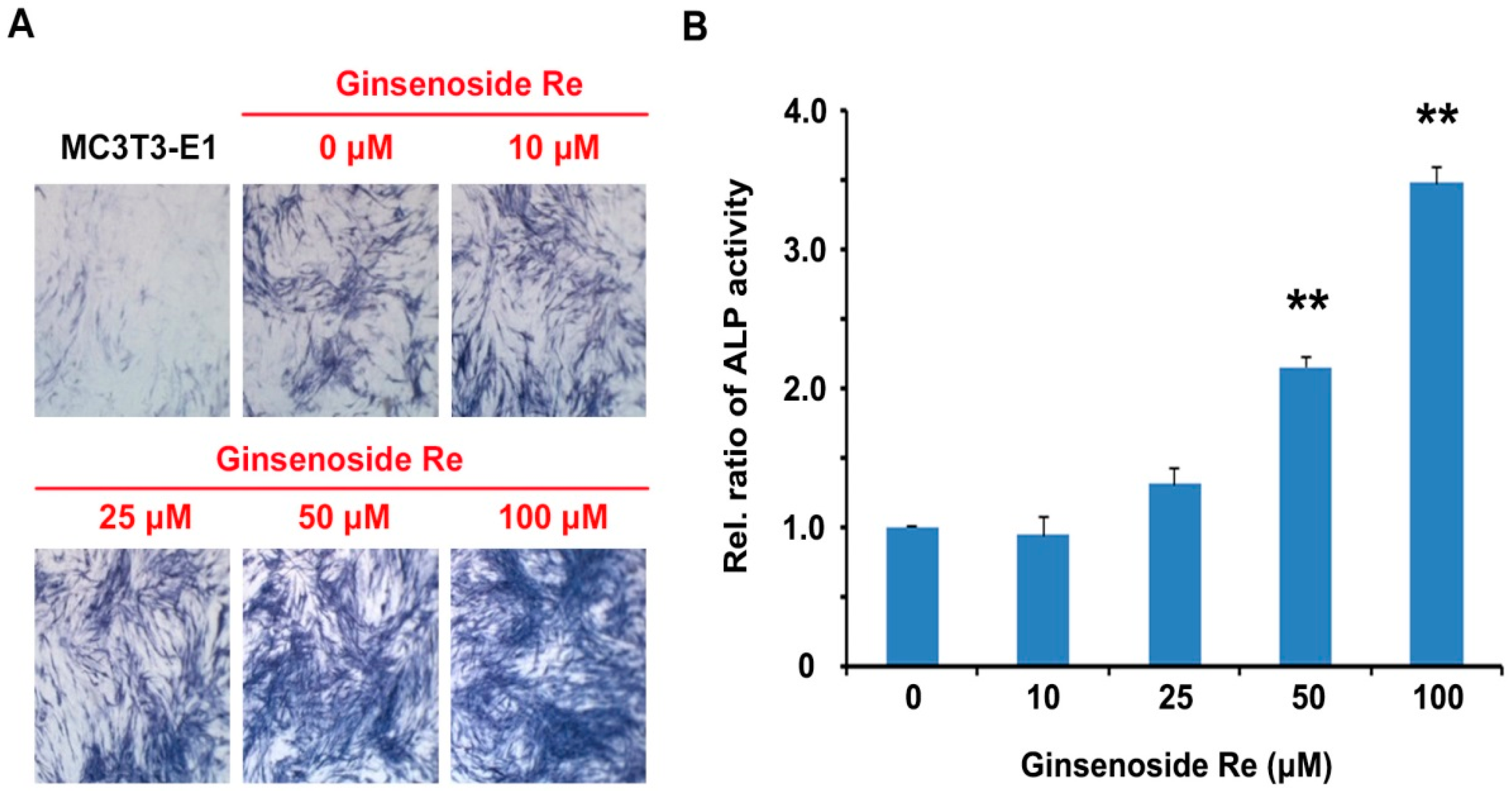
2.2. Effects of Ginsenoside Re on ALP Activity in Mouse MC3T3-E1 Cells
2.3. Effects of Ginsenoside Re on mRNA Expression of Osteoblast Differentiation Markers in MC 3T3-E1 Cells
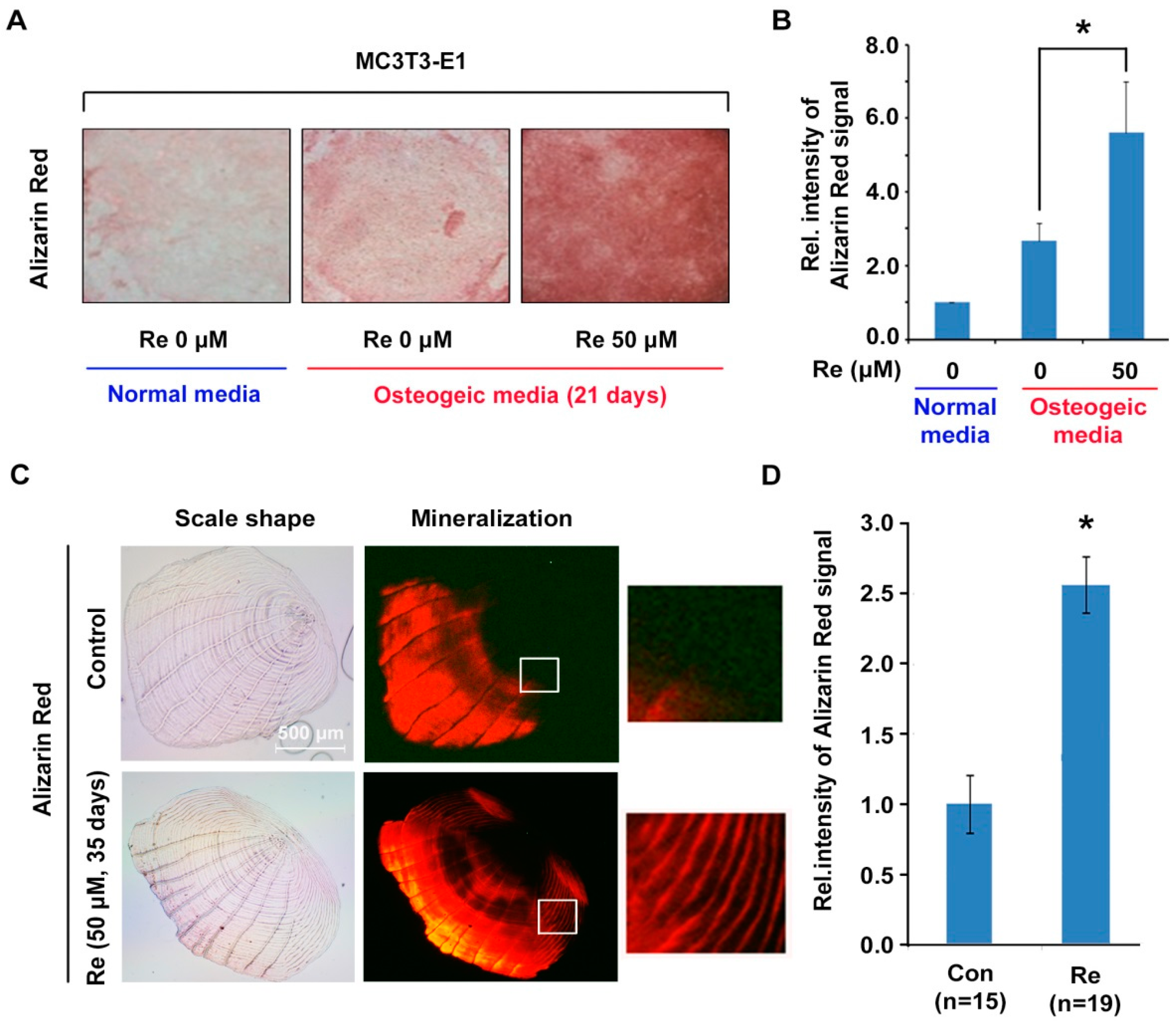
2.4. Effects of Ginsenoside Re on Mineralization in MC3T3-E1 Cells and Zebrafish Scales
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture of MC3T3-E1 Cells
3.3. MTT Assay
3.4. Alkaline Phosphatase (ALP) Enzyme Staining and Activity
3.5. Mineralization Analysis
3.6. Quantitative Real-Time RT-PCR
3.7. Zebrafish Housing
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.C.; Xu, J.; Zheng, M.H. Interaction between osteoblast and osteoclast: Impact in bone disease. Histol. Histopathol. 2004, 19, 1325–1344. [Google Scholar] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar] [PubMed]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Drugs for the management of osteoporosis: A review. Rev. Bras. Reumatol. 2011, 51, 365–371, 379–382. [Google Scholar] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.W.; Lee, J.; Oh, S.; Choi, N.K.; Park, B.J. Use of bisphosphonate and risk of atrial fibrillation in older women with osteoporosis. Osteoporos. Int. 2012, 23, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wysowski, D.K. Reports of esophageal cancer with oral bisphosphonate use. N. Engl. J. Med. 2009, 360, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Transcriptional control of skeletogenesis. Annu. Rev. Genom. Hum. Genet. 2008, 9, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar] [PubMed]
- Lee, D.C.; Yang, C.L.; Chik, S.C.; Li, J.C.; Rong, J.H.; Chan, G.C.; Lau, A.S. Bioactivity-guided identification and cell signaling technology to delineate the immunomodulatory effects of panax ginseng on human promonocytic u937 cells. J. Transl. Med. 2009, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, D.J.; Lee, M.S.; Shin, B.C.; Lee, Y.C.; Ernst, E. Red ginseng for treating erectile dysfunction: A systematic review. Br. J. Clin. Pharmacol. 2008, 66, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Xie, J.T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.Z.; Wu, J.A.; Aung, H.H.; Rue, P.A.; Bell, G.I.; et al. Anti-diabetic effect of ginsenoside re in ob/ob mice. Biochim. Biophy. Acta 2005, 1740, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Shao, Z.H.; Vanden Hoek, T.L.; Chang, W.T.; Li, J.; Mehendale, S.; Wang, C.Z.; Hsu, C.W.; Becker, L.B.; Yin, J.J.; et al. Antioxidant effects of ginsenoside re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Qu, C.; Xie, L.; Wicks, S.M.; Xie, J. Ginsenoside Re: Its chemistry, metabolism and pharmacokinetics. Chin. Med. 2012, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sun, S.; Xie, L.H.; Wicks, S.M.; Xie, J.T. Ginsenoside Re: Pharmacological effects on cardiovascular system. Cardiovasc. Ther. 2012, 30, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Kim, H.M.; Kim, D.H.; Han, H.J.; Noh, H.; Jang, J.H.; Park, S.H.; Chae, H.J.; Chae, S.W.; Ryu, E.K.; et al. Ginsenoside re inhibits osteoclast differentiation in mouse bone marrow-derived macrophages and zebrafish scale model. Mol. Cells 2016. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Protective effect of korean red ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J. Ginseng Res. 2015, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.J.; Yang, M.; Moon, C.; Kim, J.C.; Bae, C.S.; Jo, S.K.; Jang, J.S.; Kim, S.H. Effect of korean red ginseng on radiation-induced bone loss in c3h/hen mice. J. Ginseng Res. 2013, 37, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Park, S.-H.; Chae, S.-W.; Soung, N.-K.; Oh, M.-J.; Kim, J.S.; Kim, Y.O.; Chae, H.-J. Aqueous ginseng extract has a preventive role in rankl-induced osteoclast differentiation and estrogen deficiency-induced osteoporosis. J. Funct. Foods 2015, 13, 192–203. [Google Scholar] [CrossRef]
- Cheng, B.; Li, J.; Du, J.; Lv, X.; Weng, L.; Ling, C. Ginsenoside rb1 inhibits osteoclastogenesis by modulating nf-kappab and mapks pathways. Food Chem. Toxicol. 2012, 50, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fan, W.; Yin, G. The study of mechanisms of protective effect of rg1 against arthritis by inhibiting osteoclast differentiation and maturation in cia mice. Mediat. Inflamm. 2014, 2014, 305071. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Kang, S.; Noh, H.Y.; Ahn, S.; Simu, S.Y.; Aziz, M.A.; Sathishkumar, N.; Jimenez Perez, Z.E.; Yang, D.C. Inhibition of osteoclast differentiation by ginsenoside rg3 in raw264.7 cells via rankl, jnk and p38 mapk pathways through a modulation of cathepsin k: An in silico and in vitro study. Phytother. Res. PTR 2015, 29, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.J.; Veerappan, K.; Yang, D.U.; Yang, D.C. Stimulative effect of ginsenosides Rg5: Rk1 on murine osteoblastic mc3t3-e1 cells. Phytother. Res. PTR 2014, 28, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kim, Y.J.; Yang, D.C. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J. Pharm. Pharmacol. 2014, 66, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, Y.G.; Quan, H.Y.; Kim, S.J.; Jung, M.S.; Chung, S.H. Ginsenoside Rd stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells by activating amp-activated protein kinase via the BMP-2 signaling pathway. Fitoterapia 2012, 83, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.J.; Sathishkumar, N.; Yang, D.U.; Yang, D.C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng. Res. 2013, 37, 261–268. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lee, J.; Jang, J.H.; Lee, S.H.; Nan, M.H.; Oh, B.C.; Lee, S.G.; Kim, H.H.; Soung, N.K.; Ahn, J.S.; et al. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of Nf-κB, NFATc1 and c-Fos. Bone 2012, 50, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, M.S.; Park, Y.G.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside Rh2(s) induces the differentiation and mineralization of osteoblastic MC3T3-E1 cells through activation of PKD and p38 MAPK pathways. BMB Rep. 2011, 44, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, K.H.; Jung, M.S.; Huang, B.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside rh2(s) induces differentiation and mineralization of MC3T3-E1 cells through activation of the PKD/AMPK signaling pathways. Int. J. Mol. Med. 2011, 28, 753–759. [Google Scholar] [PubMed]
- Yamaguchi, A.; Komori, T.; Suda, T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and cbfa1. Endocr. Rev. 2000, 21, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos. Int. J. 2003, 14, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Scutt, A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J. Histochem. Cytochem. 2002, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, A.; Millett, P.J.; Myer, B.; Rushton, N. A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner. 1994, 27, 57–67. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Congiu, T.; Banfi, G.; Mariotti, M. Alendronate rescued osteoporotic phenotype in a model of glucocorticoid-induced osteoporosis in adult zebrafish scale. Int. J. Exp. Pathol. 2015, 96, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sire, J.Y.; Akimenko, M.A. Scale development in fish: A review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.R.; de Vrieze, E.; Lock, E.J.; Schulten, I.E.; Flik, G. Elasmoid scales of fishes as model in biomedical bone research. J. Appl. Ichthyol. 2012, 28, 382–387. [Google Scholar] [CrossRef]
- de Vrieze, E.; van Kessel, M.A.; Peters, H.M.; Spanings, F.A.; Flik, G.; Metz, J.R. Prednisolone induces osteoporosis-like phenotype in regenerating zebrafish scales. Osteoporos. Int. J. 2014, 25, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hayakawa, K.; Kameda, T.; Triba, A.; Tang, N.; Tabata, M.J.; Takada, K.; Wada, S.; Omori, K.; Srivastav, A.K.; et al. Monohydroxylated polycyclic aromatic hydrocarbons inhibit both osteoclastic and osteoblastic activities in teleost scales. Life Sci. 2009, 84, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, K.H.; Han, J.S.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C. Response of osteoblast-like cells cultured on zirconia to bone morphogenetic protein-2. J. Periodontal Implant Sci. 2011, 41, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Kim, D.H.; Han, H.-J.; Park, C.-M.; Ganipisetti, S.R.; Valan Arasu, M.; Kim, Y.O.; Park, C.G.; Kim, B.-Y.; Soung, N.-K. Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model. Molecules 2017, 22, 42. https://doi.org/10.3390/molecules22010042
Kim H-M, Kim DH, Han H-J, Park C-M, Ganipisetti SR, Valan Arasu M, Kim YO, Park CG, Kim B-Y, Soung N-K. Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model. Molecules. 2017; 22(1):42. https://doi.org/10.3390/molecules22010042
Chicago/Turabian StyleKim, Hye-Min, Dong Hyun Kim, Ho-Jin Han, Chan-Mi Park, Srinivas Rao Ganipisetti, Mariadhas Valan Arasu, Young Ock Kim, Chun Geun Park, Bo-Yeon Kim, and Nak-Kyun Soung. 2017. "Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model" Molecules 22, no. 1: 42. https://doi.org/10.3390/molecules22010042
APA StyleKim, H. -M., Kim, D. H., Han, H. -J., Park, C. -M., Ganipisetti, S. R., Valan Arasu, M., Kim, Y. O., Park, C. G., Kim, B. -Y., & Soung, N. -K. (2017). Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model. Molecules, 22(1), 42. https://doi.org/10.3390/molecules22010042





