1. Introduction
Hypothyroidism is the most common pathological hormone deficiency and is more frequent in women than in men. The incidence of hypothyroidism increases with age, especially after mid-life. Populations with a higher risk of developing hypothyroidism include postpartum women [
1], individuals with a family history of autoimmune thyroid disorders [
2,
3] and patients with previous head and neck or thyroid irradiation or surgery, or other autoimmune endocrine conditions. The main treatment for hypothyroidism is supplementation with thyroxine (such as levothyroxine sodium) to achieve restoration of thyroid function [
4]. However, due to the narrow toxic-to-therapeutic ratio of thyroid hormone, thyroxine treatment can cause some adverse reactions related to excessive or increased thyroid hormone action, and include symptomatic thyrotoxicosis, subclinical thyrotoxicosis with an increased risk of bone loss [
5], and atrial tachyarrhythmias [
6]. Compared with thyroxine treatment, Traditional Chinese Medicine (TCM) has shown significant advantages.
Compound TCMs (also known as traditional Chinese formulae) have been used in China for thousands of years. TCM has accumulated more than 100,000 formulae over the past 2000 years [
7]. Haizao Yuhu Decoction (HYD) is a formula which has been used for approximately 500 years and is well-known in TCM for its efficiency in treating thyroid-related diseases. HYD was first reported by Chen Shigong in a famous surgical monograph named
Waike Zhengzong during the Ming Dynasty period. The decoction consists of 10 crude herbs, including
Glycyrrhiza uralensis Fisch (Gancao),
Sargassum pallidum (Turn.) C.Ag. (Haizao),
Ecklonia kurome Okam (Kunbu),
Pinellia ternata (Thunb.) Breit. (Banxia),
Fritillaria thunbergii Miq. (Zhebeimu),
Forsythia suspensa (Thunb.) Vahl (Lianqiao),
Ligusticum chuanxiong Hort. (Chuanxiong),
Angelica sinensis (Oliv.) Diels. (Danggui),
Angelica pubescens Maxim.f.
biserrata Shan et Yuan (Duhuo) and
Citrus reticulata Blanco (Chenpi and Qingpi). Modern research has shown that HYD exhibits significant protective effects by reversing serum T
3, T
4, TgAb and TPOAb levels, histopathological changes and TRAIL protein expression in the thyroid in rat models of experimental autoimmune thyroiditis (EAT) [
8]. HYD with different compatibility can inhibit thyroid enlargement induced by propylthiouracil (PTU), to some extent, and the mechanism may be related to the activation of gene transcription of thyroid peroxidase (TPO) and thyroid globulin [
9].
However, the clinical application of HYD is limited due to the Haizao and Gancao drug combination which is considered to be an unfavorable combination according to the “Eighteen antagonisms” (also known as Shibafan in Chinese) which are controversially prohibited combinations in TCM, based on a history of thousands of years of experience. Pharmacokinetic studies of many formulae have reported concerns regarding the drug combinations used in orally administered traditional Chinese recipes [
10,
11] in terms of their curative effect and the rationality of these drug combinations used in the formulae. In the present study, we aim to develop an effective method of enhancing the level of the Haizao-Gancao drug combination by comparing pharmacokinetic studies of different prescriptions of HYD containing eleven compounds, including liquiritin (
1), naringin (
2), hesperidin (
3), peimine (
4), peiminine (
5), liquiritigenin (
6), glycyrrhizic acid (
7), bergapten (
8), nobiletin (
9), osthole (
10), and glycyrrhetinic acid (
11) (
Figure 1). Information about the 11 compounds is shown in
Table 1.
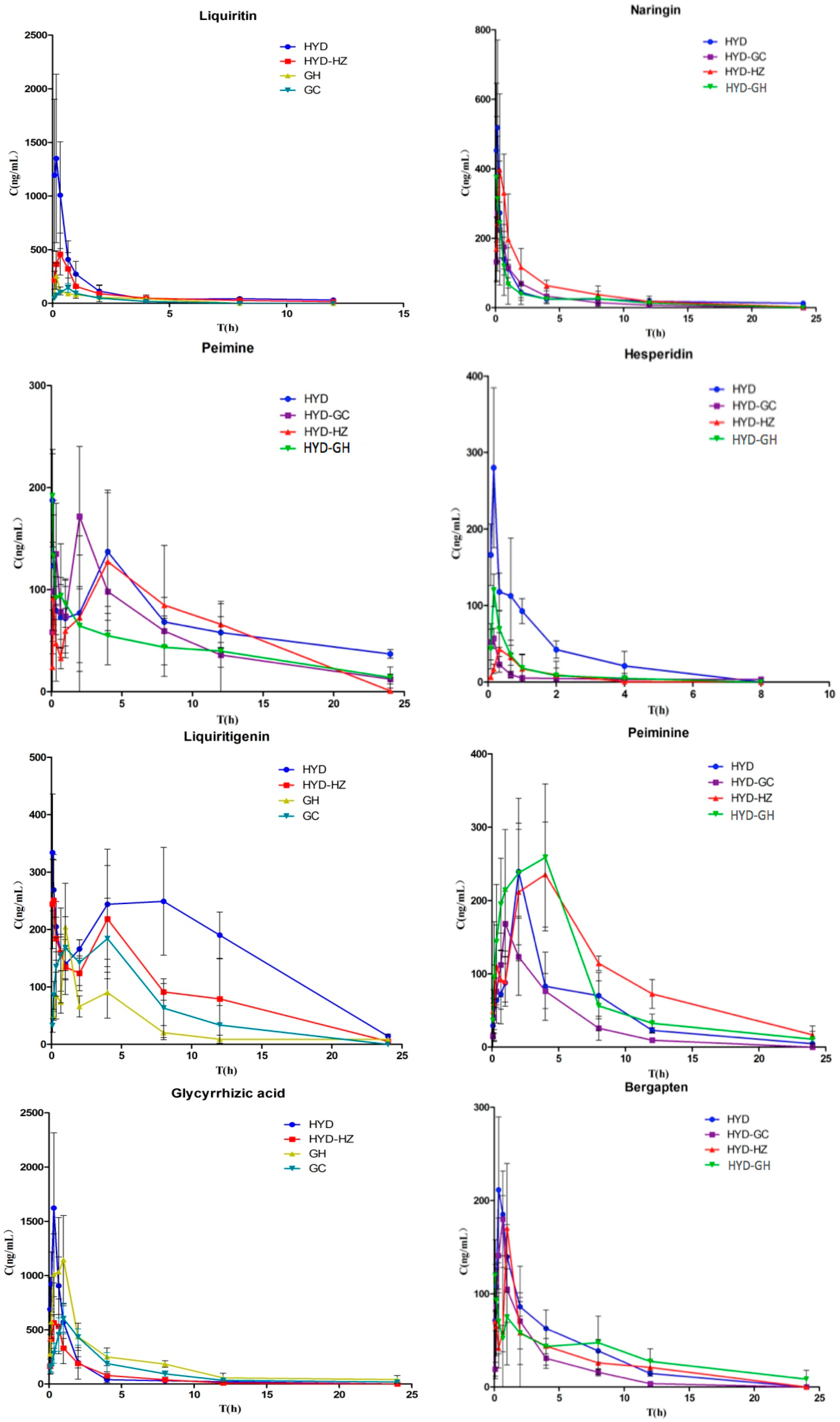
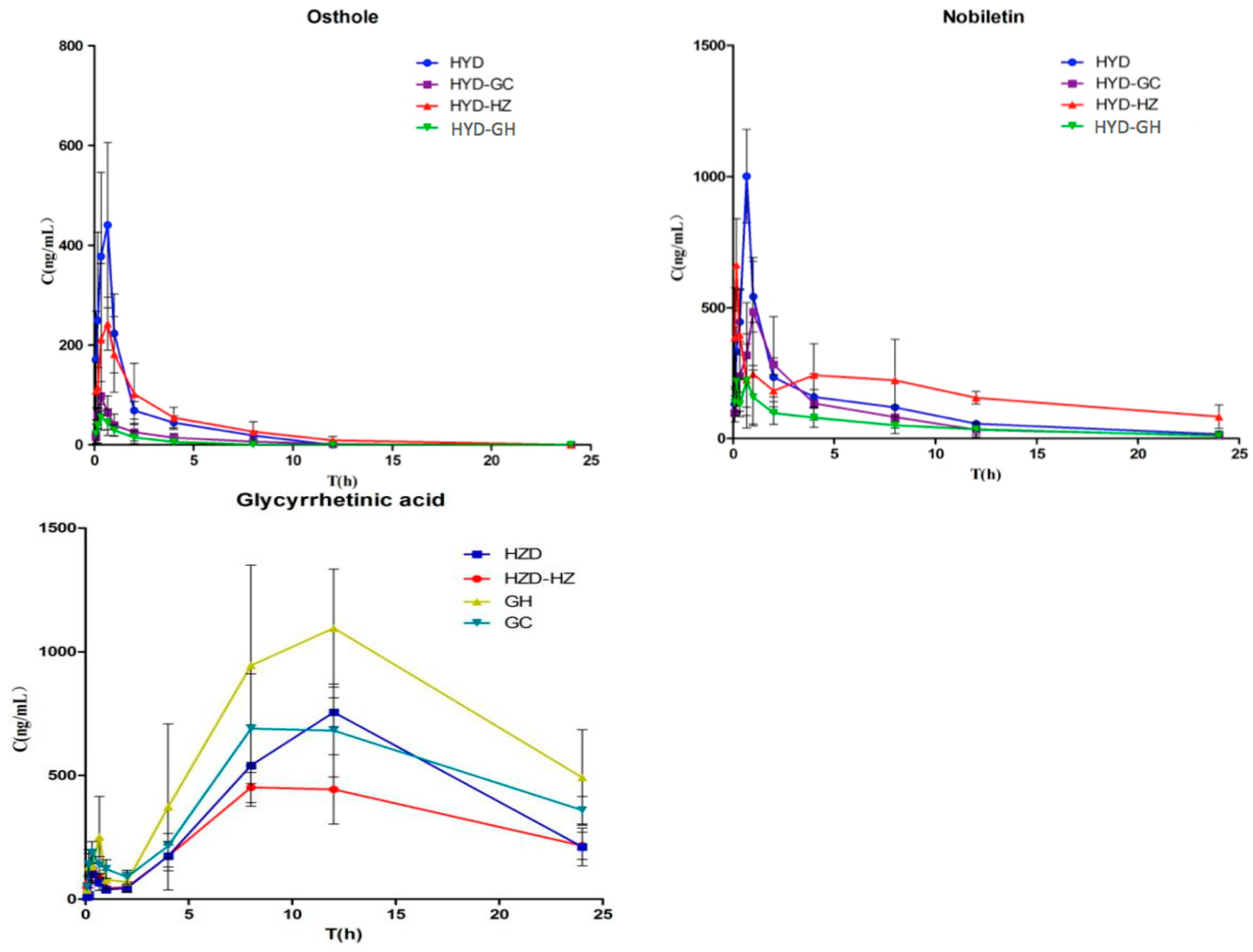
In this study, a rapid liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed for the determination of glycyrrhizinic acid, liquiritin, liquiritigenin, glycyrrhetic acid, hesperidin, naringin, nobiletin, peimine, peiminine, osthole, and bergapten after oral administration of HYD plus-minus Haizao and Gancao drug combination. In addition, pharmacokinetic profile differences in the different prescriptions of HYD in rats were investigated in order to determine the compatibility of the Haizao and Gancao drug combination and other herbs in HYD.
3. Experimental Section
3.1. Materials and Reagents
HYD includes the following crude drugs: (1) Glycyrrhiza uralensis Fisch (Gancao); (2) Sargassum pallidum (Turn.) C.Ag. (Haizao); (3) Ecklonia kurome Okam (Kunbu); (4) Pinellia ternata (Thunb.) Breit. (Banxia), (5) Fritillaria thunbergii Miq. (Zhebeimu); (6) Forsythia suspensa (Thunb.) Vahl (Lianqiao); (7) Ligusticum chuanxiong Hort. (Chuanxiong); (8) Angelica sinensis (Oliv.) Diels. (Danggui); (9) Angelica pubescens Maxim.f. biserrata Shan et Yuan (Duhuo); and (10) Citrus reticulata Blanco (Chenpi and Qingpi). All materials were purchased from the Fengyuan Pharmaceutical Company of Anhui Province (Hefei, China) and authenticated by Jinao Duan. These materials met the qualitative and quantitative stipulations of the 2010 Chinese Pharmacopoeia. Voucher specimens were deposited in the Herbarium of Nanjing University of Chinese Medicine, Nanjing, China. Acetonitrile and formic acid were of HPLC-grade and obtained from Merck (Darmstadt, Germany) and deionized water was purified using an EPED super purification system (Eped, Nanjing, China). The reference compounds liquiritin (111610-201106), naringin (110722-201312), hesperidin (110721-2001316), peimine (110750-201110), peiminine (110751-201110) and osthole (110822-201308) were purchased from the Chinese National Institute of Pharmaceutical and Biological Products (Beijing, China). Glycyrrhizic acid, glycyrrhetinic acid, nobiletin and bergapten were purchased from Nanjing Spring-Autumn Biological Engineering Co., Ltd. (Nanjing, China). Liquiritigenin (131229) was purchased from Shanghai Winherb Medical Technology Co., Ltd. (Shanghai, China). All other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. (Nanjing, China), unless otherwise stated.
3.2. Animals
All experiments were performed with male Wistar rats, weighing 220–250 g, obtained from the Vital River Experimental Animal Co., Ltd. (Beijing, China). They were kept in plastic cages at 22 ± 2 °C with free access to pellet food and water. Animal welfare and experimental procedures were carried out in accordance with the guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, USA), and the Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) and related ethical regulations of Nanjing University of Chinese Medicine.
3.3. Chromatographic Conditions
Chromatographic analysis was performed using a Waters Acquity UPLC system (Waters Corp., Milford, MA, USA), consisting of a binary pump solvent management system, an online degasser, and an auto-sampler. An Acquity UPLC BEH C 18 (100 mm × 2.1 mm, 1.7 μm) column was used for all analyses. The column temperature was maintained at 35 °C. The mobile phase was composed of (A) formic acid aqueous solution (0.1%); and (B) acetonitrile using a gradient elution of 10%–30% B at 0–4 min, 30%–80% B at 5–9 min, 80%–95% B at 5–9 min, 95% B at 9–10 min, 95%–100% B at 10–11.2 min.
3.4. Mass Spectrometry Conditions
Mass spectrometry detection was performed using a Xevo Triple Quadrupole MS (Waters Corp.) equipped with an electro-spray ionization source (ESI). The ESI source was set in both positive and negative ionization mode. The parameters in the source were set as follows: capillary voltage 3.0 kV; source temperature 150 °C; desolvation temperature 550 °C; cone gas flow 50 L/h; desolvation gas flow 1000 L/h. Analyte detection was performed using MRM. The cone voltage and collision energy were optimized for each analyte and selected values are shown in
Table 10. All data collected in centroid mode were acquired using Masslynx 4.1 software (Waters Corp.) and post-acquisition quantitative analysis was performed using the TargetLynx program (Waters Corp.).
3.5. Preparation of HYD and Omitted Ingredients in HYD
Raw materials of Glycyrrhiza uralensis Fisch (Gancao) (200 g) were crushed into small pieces and then refluxed with 2 L water for 1 h and with 1.6 L water for 1 h. The filtrates were combined and concentrated below 70 °C to obtain a certain volume at the ratio of 2:1 (w/w, weight of all constituting herbs and the extract filtrates) under vacuum. The same method was used to prepare the extract of Sargassum pallidum (Turn.) C.Ag. (Haizao), Ecklonia kurome Okam (Kunbu), Pinellia ternata (Thunb.) Breit. (Banxia), Fritillaria thunbergii Miq. (Zhebeimu), Forsythia suspensa (Thunb.) Vahl (Lianqiao), Ligusticum chuanxiong Hort. (Chuanxiong), Angelica sinensis (Oliv.) Diels. (Danggui), Angelica pubescens Maxim.f. biserrata Shan et Yuan (Duhuo) and Citrus reticulata Blanco (Chenpi and Qingpi). A 10 mL extract of Gancao, Haizao, Banxia, Zhebeimu, Lianqiao, Chuanxiong, Danggui, Duhuo, Chenpi, Qingpi and a 5 mL extract of Kunbu were mixed together to prepare the HYD extract. HYD minus Gancao (HYD-GC); HYD minus Haizao (HYD-HZ); HYD minus Gancao and Haizao (HYD-GH); the extract of Gancao and Haizao (GH) and the extract of Gancao (GC) were also prepared. The extracts contained 13.87, 11.73, 16.53, 9.87, 13.84, 11.96, 28.14, 16.94, 10.09, 17.41, 4.19 μg/mL of compounds 1–11 in HYD; 0, 11.73, 16.53, 9.87, 13.84, 0, 0, 16.94, 10.09, 17.41, 0 μg/mL of compounds 1–11 in HYD-GC; 13.87, 11.73, 16.53, 9.87, 13.84, 11.96, 28.14, 16.94, 10.09, 17.41, 4.19 μg/mL of compounds 1–11 in HYD-HZ; 0, 11.73, 16.53, 9.87, 13.84, 0, 0, 16.94, 10.09, 17.41, 0 μg/mL of compounds 1–11 in HYD-GH; 13.87, 0, 0, 0, 0, 11.96, 28.14, 0, 0, 0, 4.19 μg/mL of compounds 1–11 in GH, and 13.87, 0, 0, 0, 0, 11.96, 28.14, 0, 0, 0, 4.19 μg/mL of compounds 1–11 in GC.
3.6. Preparation of Calibration Standards and Quality Control Samples
A standard stock solution mixture containing the eleven compounds was prepared in methanol with a final concentration of 28.2 μg/mL for liquiritin (1); 22.7 μg/mL for naringin (2); 18.7 μg/mL for hesperidin (3); 17.7 μg/mL for peimine (4); 18.2 μg/mL for peiminine (5); 21.1 μg/mL for liquiritigenin (6); 22.2 μg/mL for glycyrrhizic acid (7); 21.4 μg/mL for bergapten (8); 18.1 μg/mL for nobiletin (9); 18.5 μg/mL for osthole (10); and 24.5 μg/mL for glycyrrhetinic acid (11), respectively. The stock solution was serially diluted with methanol to provide working standard solutions of the desired concentrations. The IS stock solutions (24.6 μg/mL for diphenhydramine—IS for positive ionization mode and 23.8 μg/mL for chloromycetin—IS for negative ionization mode) were also prepared using methanol. IS working solutions (1215 ng/mL for diphenhydramine, 476 ng/mL for chloromycetin) were prepared by diluting the stock solution with methanol. Calibration samples were prepared by mixing solutions of standard mixture, IS and methanol with rat blank plasma to obtain final concentrations in the range of 2.82–2820 ng/mL for liquiritin, 2.27–2270 ng/mL for naringin, 1.87–1870 ng/mL for hesperidin, 1.77–1770 ng/mL for peimine, 1.82–1820 ng/mL for peiminine, 2.11–2110 ng/mL for liquiritigenin, 2.22–2220 ng/mL for glycyrrhizic acid, 2.14–2140 ng/mL for bergapten, 1.81–1810 ng/mL for nobiletin, 1.85–1850 ng/mL for osthole, 1.82–1820 ng/mL for glycyrrhetinic acid, and 1215 ng/mL for diphenhydramine (IS for positive ionization mode) and 476 ng/mL for chloromycetin (IS for negative ionization mode) for IS, respectively. All solutions were stored at −20 °C before use. QC samples were also prepared in the same way (2.82, 141, 2820 ng/mL for liquiritin, 2.27, 114, 1135 ng/mL for naringin, 1.87, 94, 935 ng/mL for hesperidin, 1.77, 88.5, 1770 ng/mL for peimine, 1.82, 91, 910 ng/mL for peiminine, 2.11, 105.5, 1055 ng/mL for liquiritigenin, 2.22, 111, 2220 ng/mL for glycyrrhizic acid, 2.14, 107, 1070 ng/mL for bergapten, 1.81, 90.5, 1810 ng/mL for nobiletin, 1.85, 92.5, 925 ng/mL for osthole and 2.45, 122.5, 2450 ng/mL for glycyrrhetinic acid) at low, middle and high concentrations.
3.7. Validation Procedures
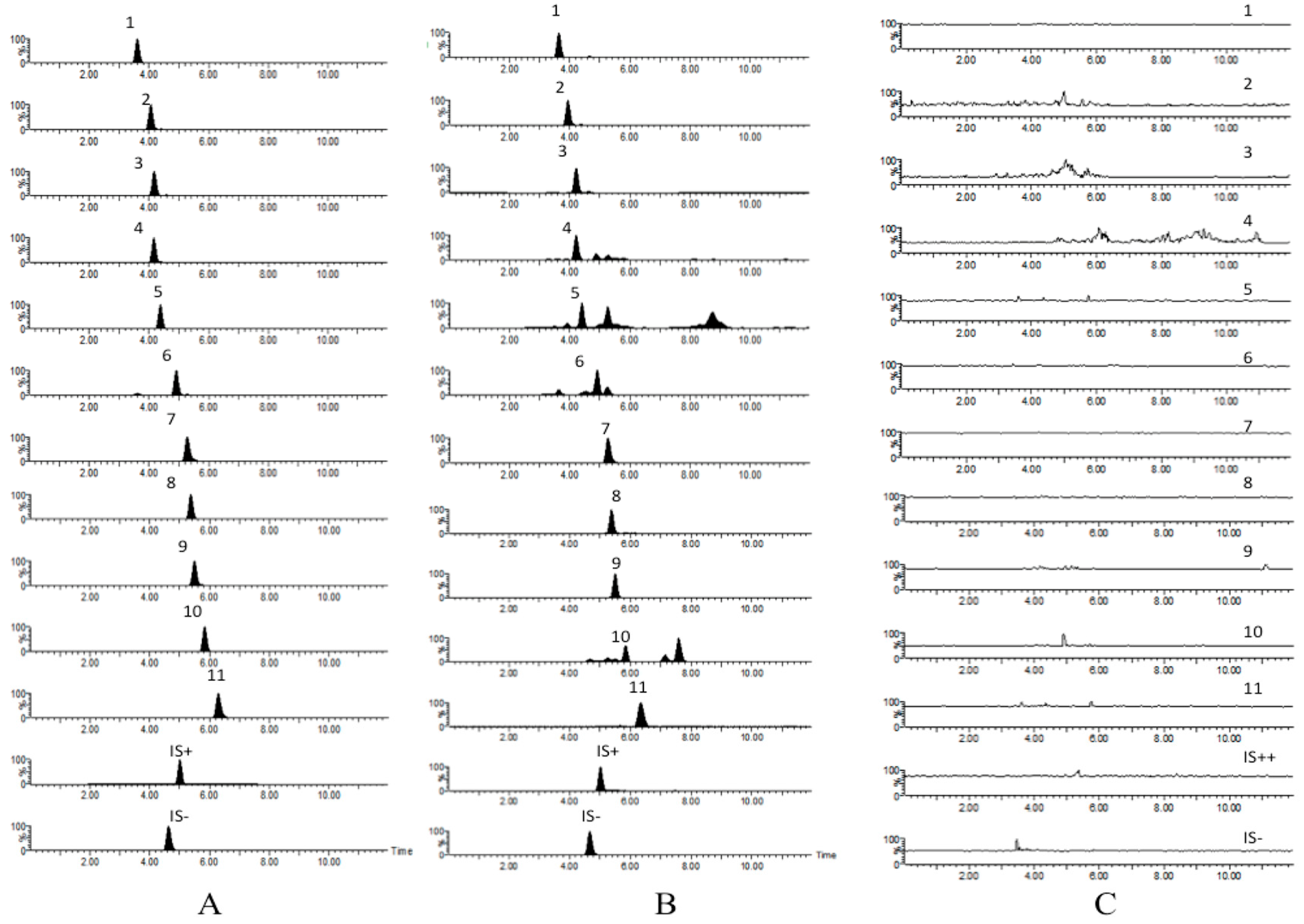
The specificity of the method was evaluated by preparing and analyzing six different batches of rat plasma to determine potential interferences at the LC peak region for analytes and IS. The rat plasma chromatograms were compared with those obtained with a sample at the concentration of LLOQ. The signal intensity at this concentration was at least five times higher than that of blank plasma.
The linearity of each calibration curve was determined by plotting the peak area ratio (y) of analytes to IS versus the nominal concentration (x) of analytes with weighted (1/x2) least square linear regression.
Accuracy and intra- and inter-day precision were estimated by analyzing three QC samples (five samples for each) at low, middle and high concentrations on the same day and on three consecutive validation days, respectively. The precision was evaluated by relative standard deviation (RSD %) and accuracy by (mean measured concentration/spiked concentration) × 100%.
Extraction recovery was assessed by comparing the peak responses of three QC samples (five samples for each) with the responses of analytes from standard solutions spiked in post-extracted blank plasma at equivalent concentrations.
The matrix effect was measured by comparing the peak responses obtained from samples where the extracted matrix was spiked with standard solutions to those obtained from neat standard solutions at equivalent concentrations.
Three QC samples (five samples for each) were tested for pre-treatment, post-treatment, three freeze-thaw cycles and long-term stabilities. Pre-treatment stability was assessed by exposing QC samples to room temperature for 4 h. Post-treatment stability was evaluated by placing QC samples in the auto-sampler at 4 °C for 24 h. For freeze-thaw cycle stability assessment, QC samples were repeatedly frozen and thawed for three cycles from −80 °C to 20 °C. Long-term stability was carried out by placing QC samples at −80 °C for 2 weeks.
3.8. Preparation of Rat Model of Hypothyroidism
A considerable increase in thyroid stimulating hormone (TSH) and decrease in triiodothyronine (T
3) and tetraiodothyronine (T
4) in serum are considered to be markers of successful hypothyroid rat model preparation [
30]. PTU is the classic drug used to replicate hypothyroidism in rat models. It is generally thought that PTU can decrease the level of thyroid hormone in a hypothyroid rat model by inhibiting the activity of TPO [
31,
32]. Model Wistar rats were administered PTU intragastrically at a dose of 10 mg/kg (QD) for 2 weeks to establish hypothyroidism. Serum from model rats was collected on the last day of PTU administration and the T
3, T
4 and TSH levels were determined using test kits. Wistar rats with a considerable increase in thyroid TSH and a decrease in serum T
3 and T
4 were considered to have hypothyroidism and were selected for subsequent pharmacokinetic studies. The levels of these hormones are shown in
Table 11.
3.9. Pharmacokinetic Studies
For the pharmacokinetic studies, model Wistar rats were divided into six groups (n = 6 per group). Rats in the model HYD groups were administered HYD at a dose of 12 mL/kg intragastrically. In the model HYD-GC groups, HYD-GC (11 mL/kg) was administered. In the model HYD-HZ groups, HYD-HZ (11 mL/kg) was administered. In the model HYD-GC-HZ groups, HYD-GC-HZ (10 mL/kg) was administered. In the model GH groups, GH (2 mL/kg) was administered. In the model GC groups, GC (1 mL/kg) was administered. Blood samples were collected at specific time points before (0 min) and after oral administration (5, 10, 20, 40, 60, 120, 240, 480, 720, 1440 min). A total of 360 blood samples were collected. All blood samples were immediately centrifuged at 2500 rpm for 10 min to obtain plasma, which was labeled and frozen at −80 °C until analysis. Blank plasma was obtained from the rats without oral administration and was used to investigate the assay development and validation.
3.10. Statistical Analysis
To determine the pharmacokinetic parameters of compounds 1–11 in different groups, concentration–time data were analyzed by DAS 3.2 software (Mathematical Pharmacology Professional Committee of China, Shanghai, China, 2011). Data were expressed as the mean and standard deviation (S.D.) with triplicate measurements. The identification of significant differences between different groups was carried out using the Student’s t-test. A p value < 0.05 was considered statistically significant.