Kaempferol and Chrysin Synergies to Improve Septic Mice Survival
Abstract
:1. Introduction
2. Results
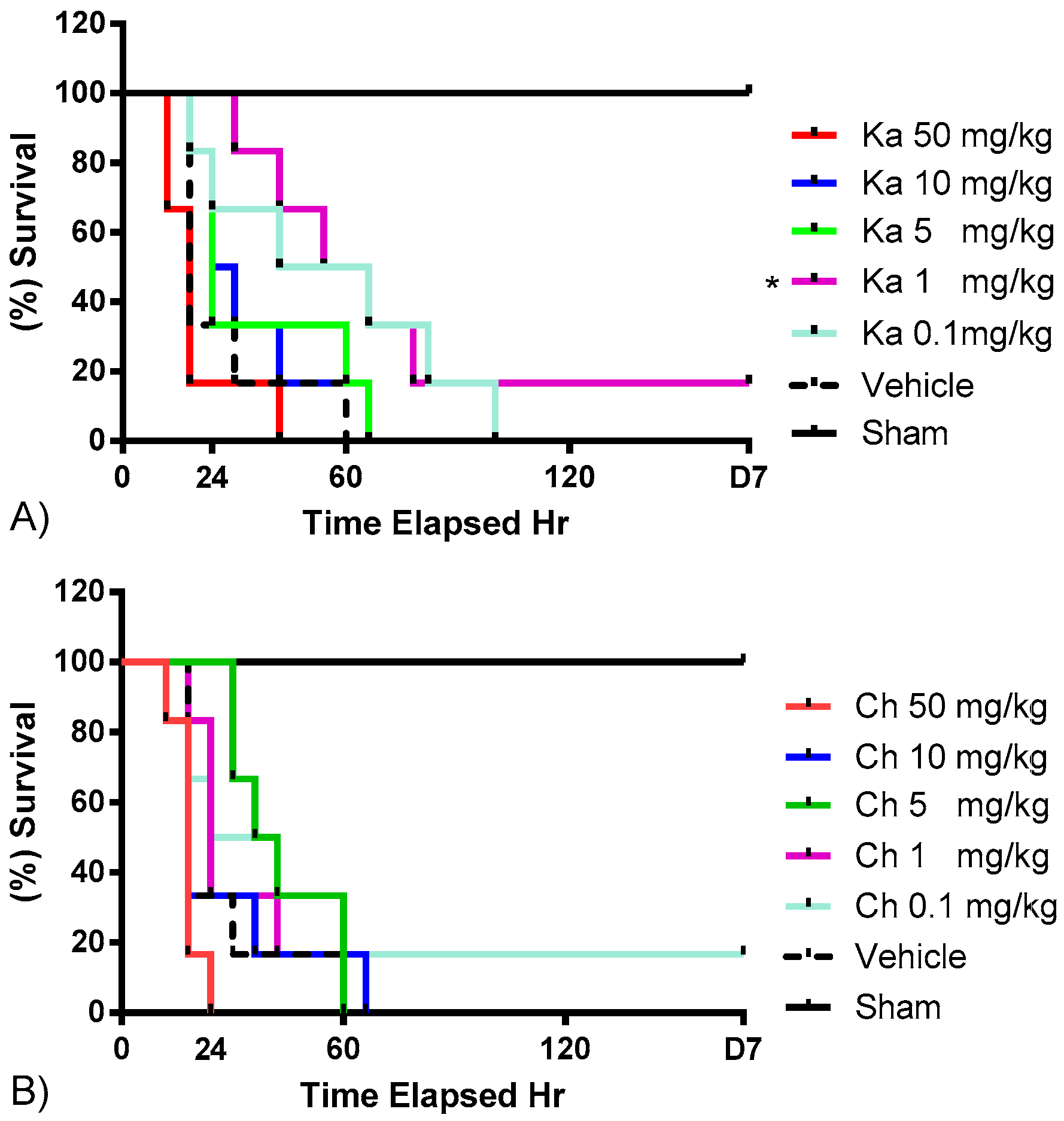
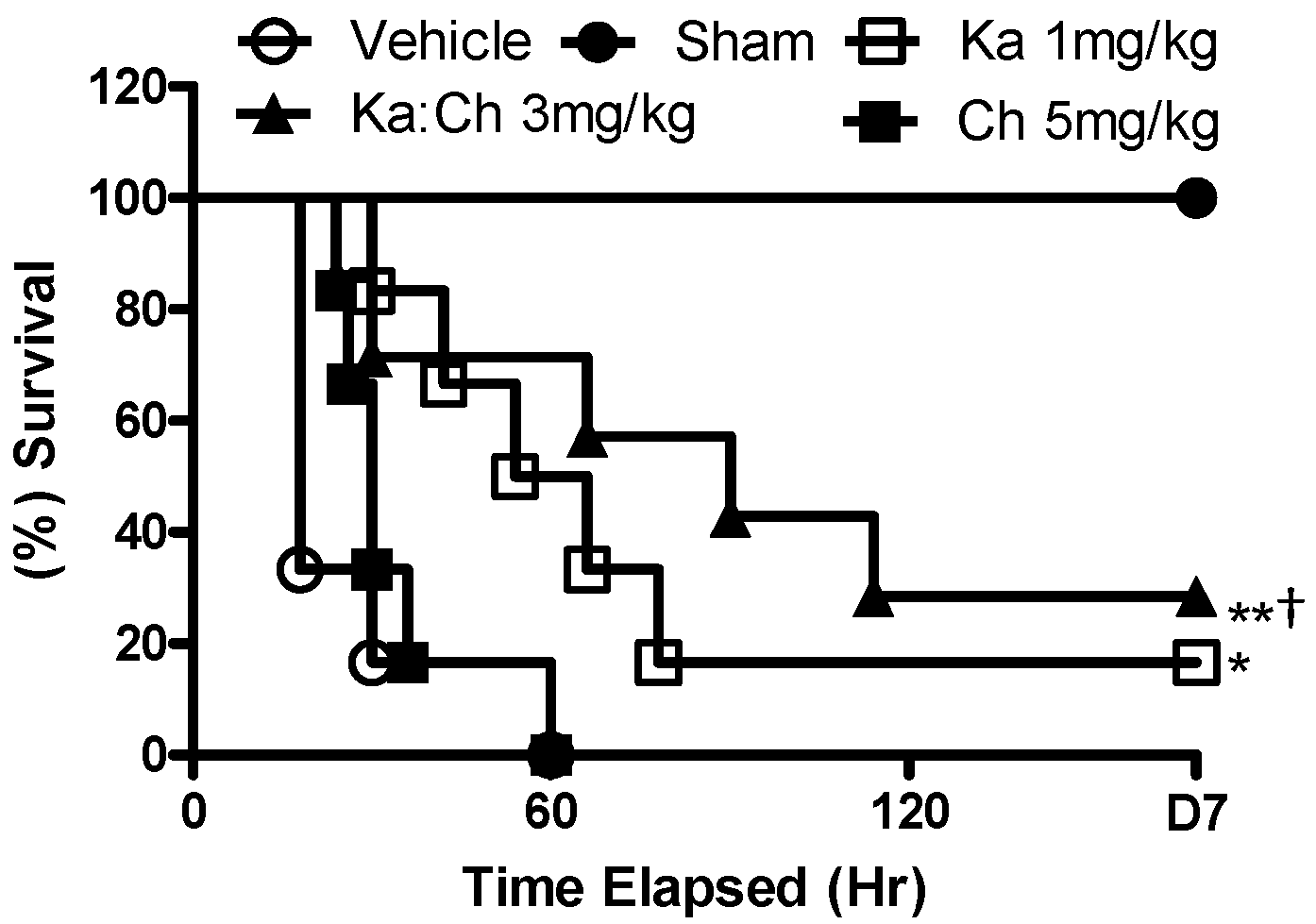
2.1. Effects of Chrysin and Kaempferol Individual or Combination Treatments on Endotoxin Shock
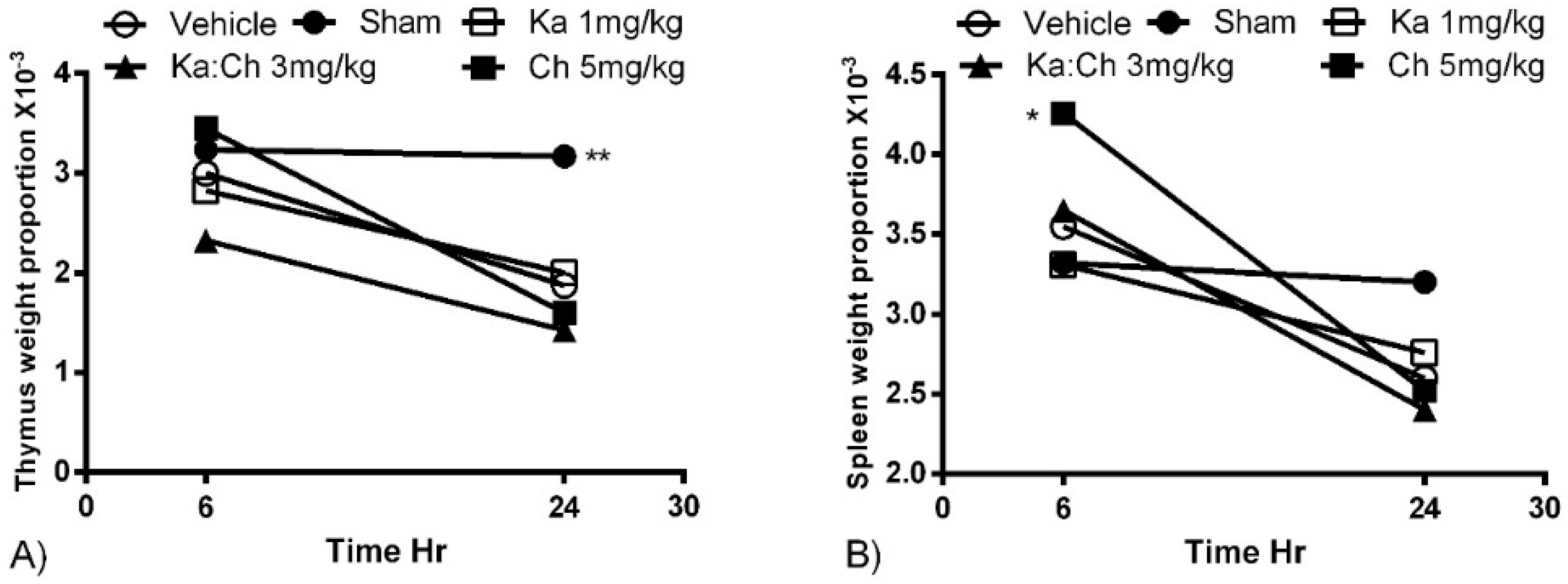
2.2. Organ Weights
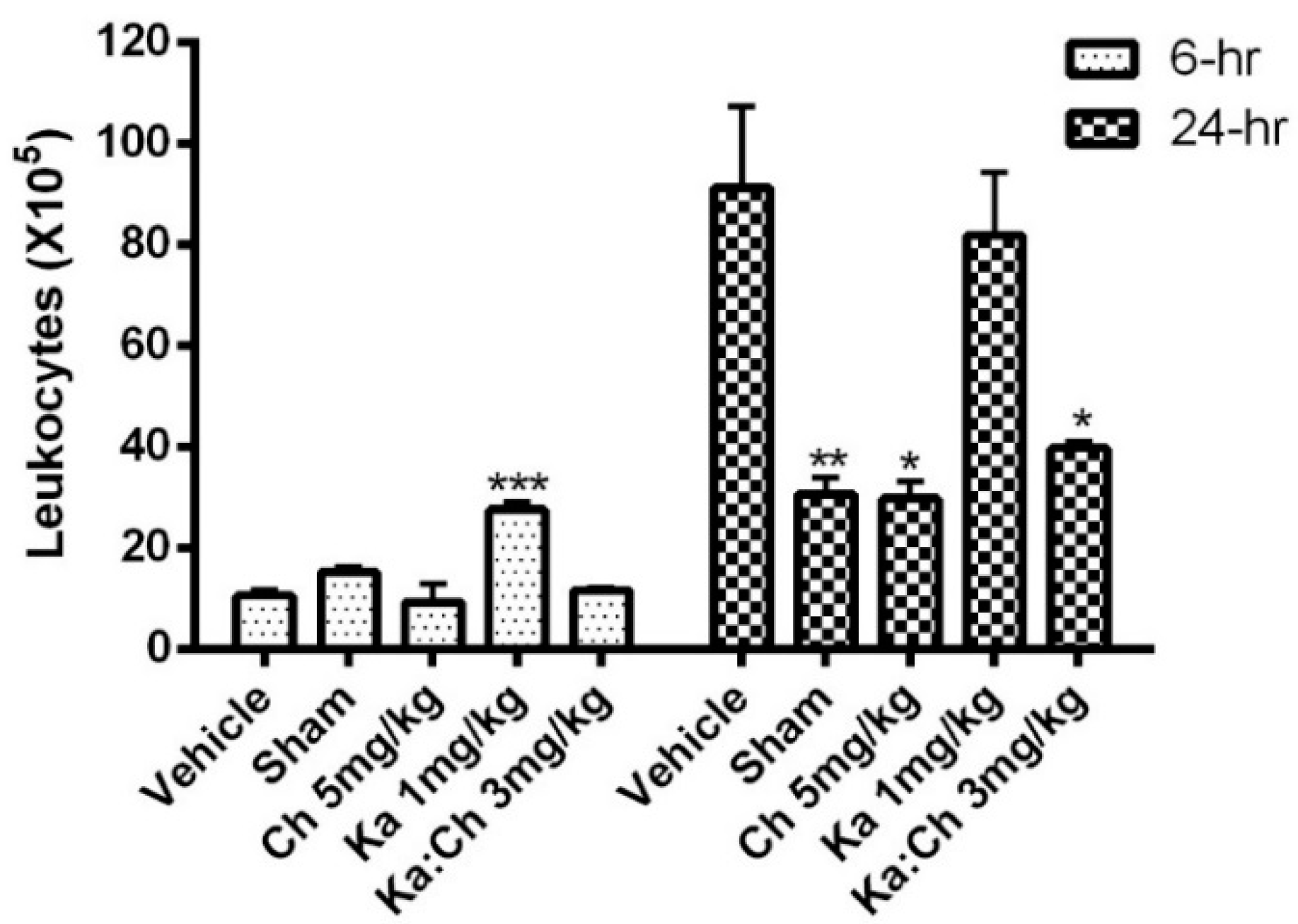
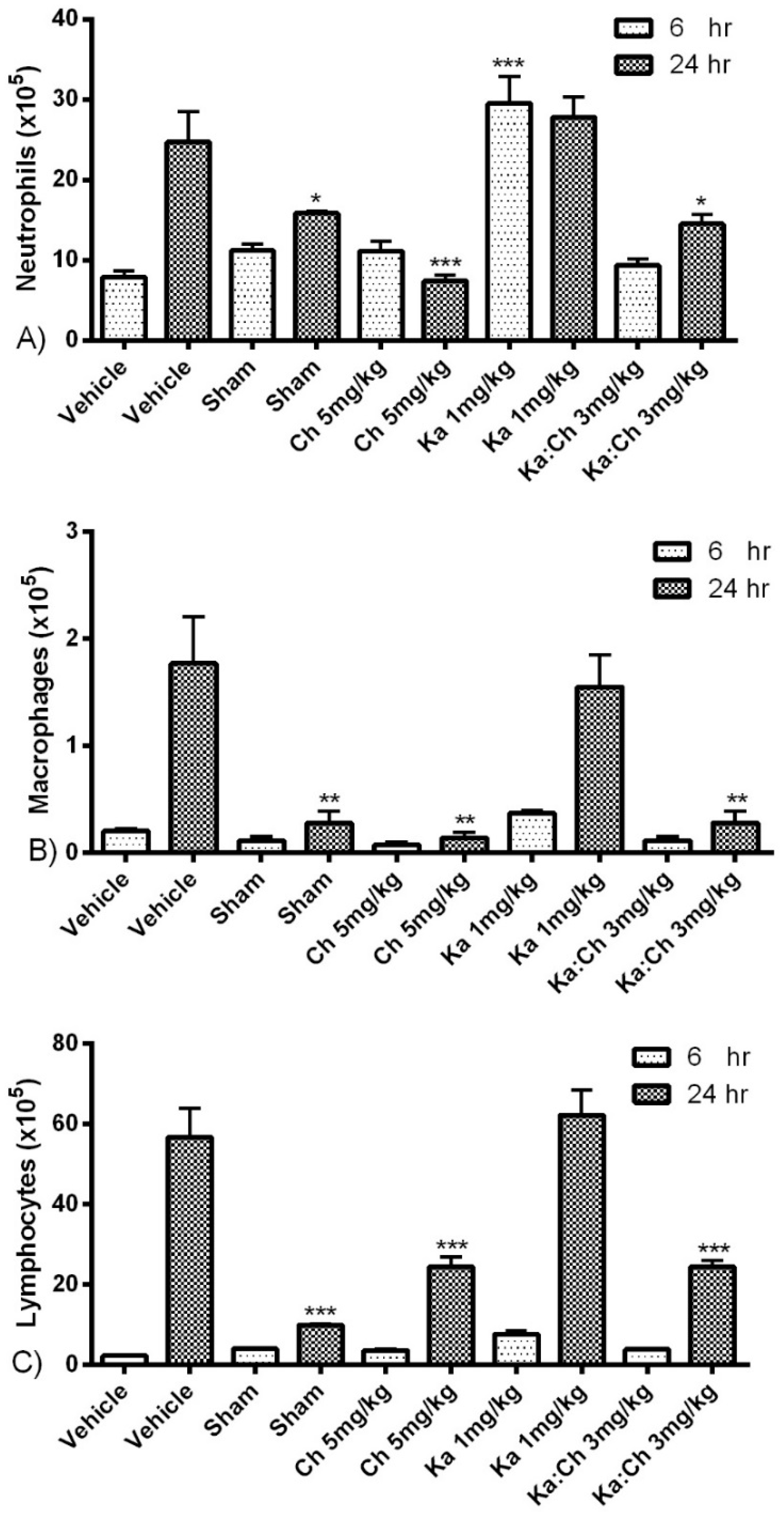
2.3. Quantitative and Differential Leukocytic Count
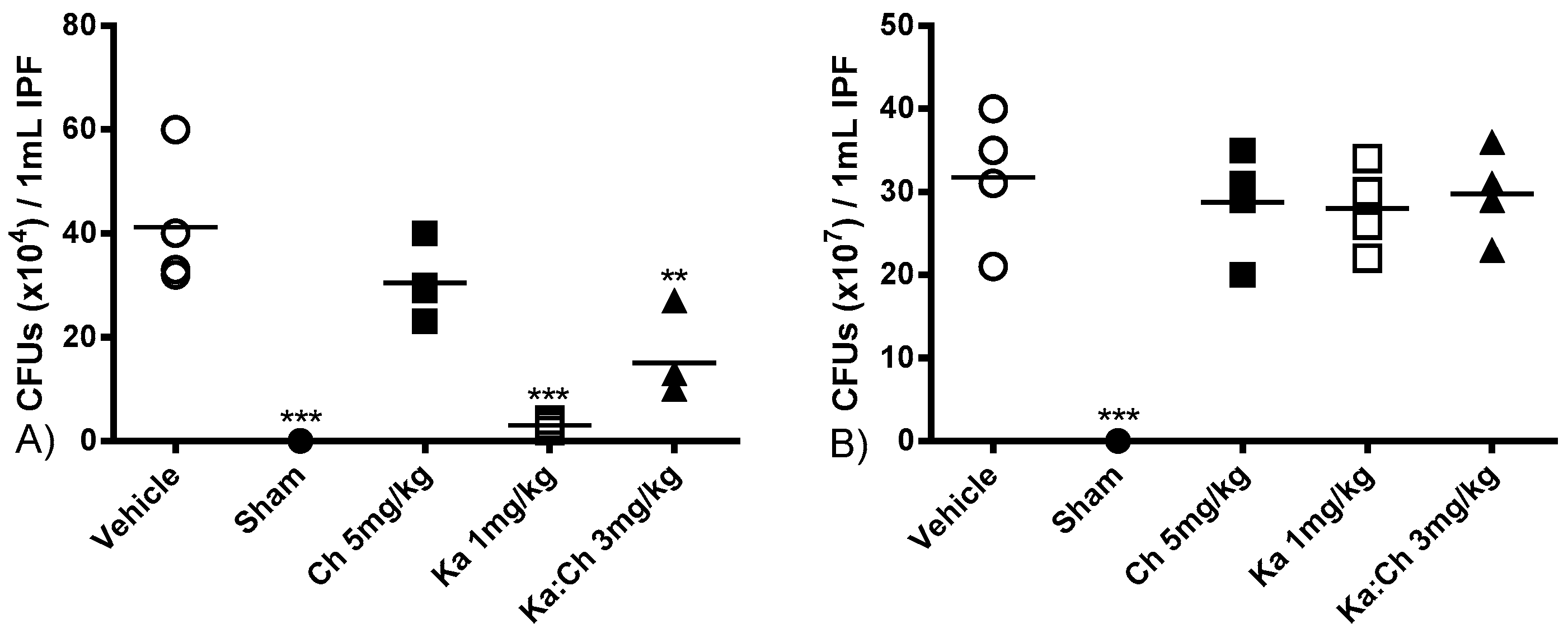
2.4. Bacterial Clearance
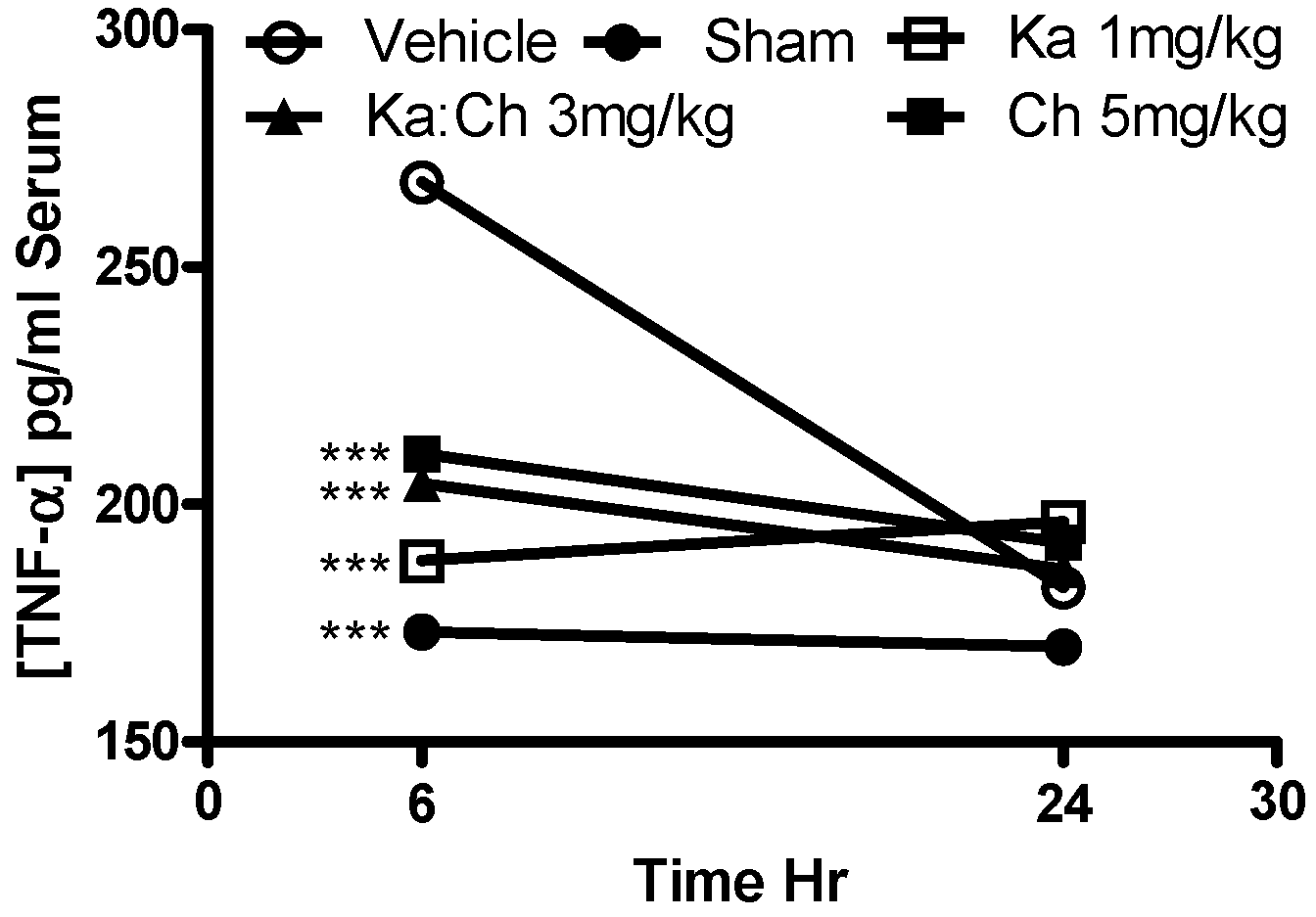
2.5. Serum AST and TNF-α Levels
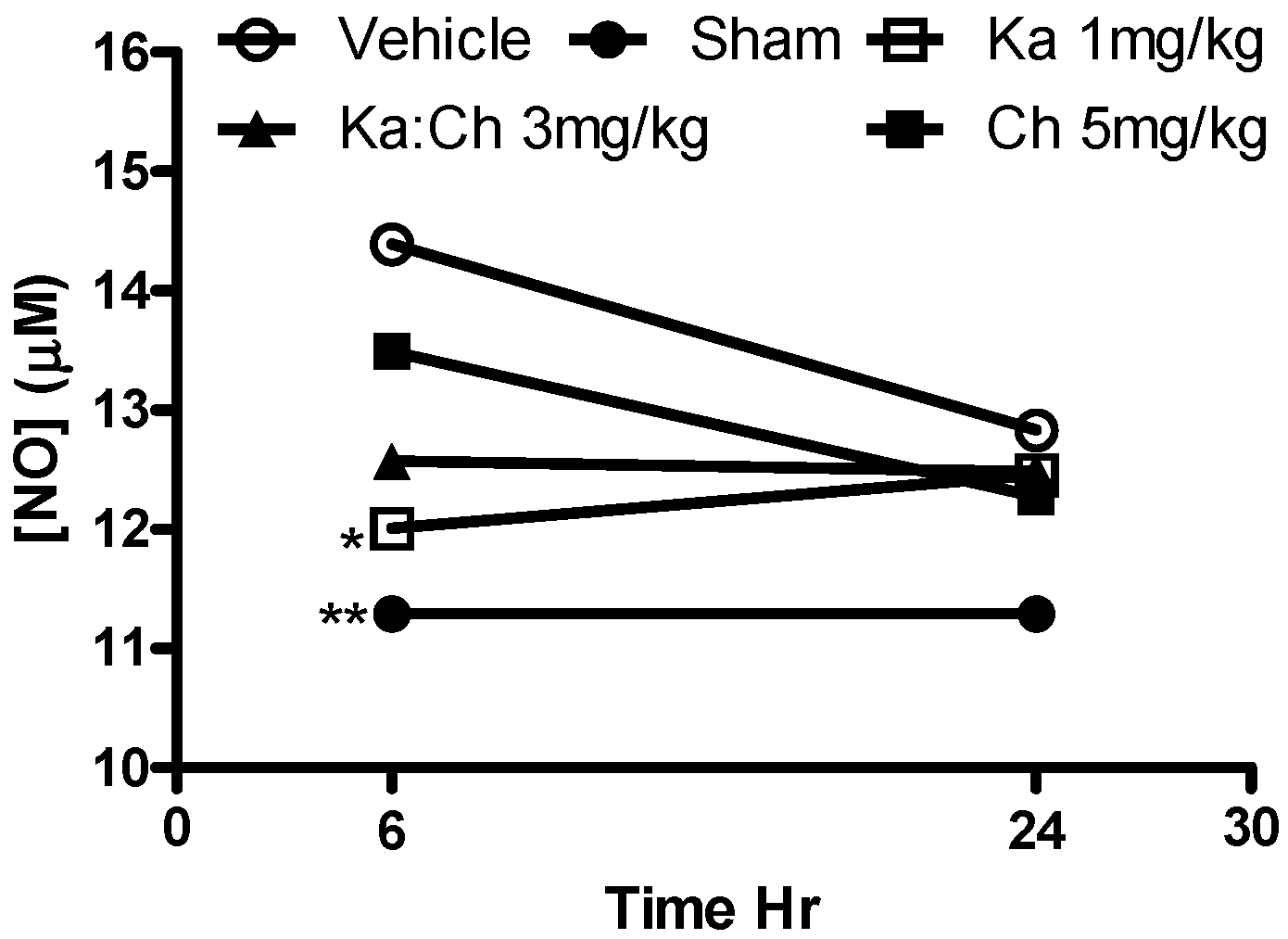
2.6. Nitrite Determination
2.7. MPO Activity in Liver Tissue
3. Statistical Analysis
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol and Combination
4.3. CLP as a Sepsis Model
4.4. Survival Rates, Harvesting Samples, and Cytokine Determination
4.5. Serum Nitrite Determination
4.6. Measurement of MPO Activity in Liver Tissue
5. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulloa, L.; Tracey, K.J. The ‘cytokine profile’: A code for sepsis. Trends Mol. Med. 2005, 11, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Shao, D.; Liu, J.; Zhu, J.; Zhang, Z.; Xu, J. Effects of ketamine on levels of cytokines, NF-κB and TLRs in rat intestine during CLP-induced sepsis. Int. Immunopharmacol. 2007, 7, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.B.; De Maio, A. An Exaggerated Inflammatory Response after CLP Correlates with a Negative Outcome. J. Surg. Res. 2005, 125, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, P.; Hoegl, S.; Revermann, M.; Ahluwalia, D.; Zander, J.; Boost, K.A.; Nguyen, T.; Zwissler, B.; Muhl, H.; Hofstetter, C. Cecal Ligation and Incision: An Acute Onset Model of Severe Sepsis in Rats. J. Surg. Res. 2009, 151, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-Q.; Shinozawa, Y.; Sasaki, J.; Takuma, K.; Akaishi, S.; Yamanouchi, S.; Endo, T.; Nomura, R.; Kobayashi, M.; Kudo, D.; et al. The Effects of Arginine and Selective Inducible Nitric Oxide Synthase Inhibitor on Pathophysiology of Sepsis in a CLP Model. J. Surg. Res. 2008, 146, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Chung, F.T.; Kuo, C.H.; Chou, P.C.; Wang, T.Y.; Chang, P.J.; Lo, Y.L.; Huang, C.D.; Lin, H.C.; Wang, C.H.; et al. Circulating angiopopietin-1 correlates with the clinical course of multiple organ dysfunction syndrome and mortality in patients with severe sepsis. Medicine 2015, 94, e878. [Google Scholar] [CrossRef] [PubMed]
- Seam, N.; Suffredini, A.F. Mechanisms of sepsis and insights from clinical trials. Drug Discov. Today Dis. Mech. 2007, 4, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Canini, A. Chrysin-Induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int. J. Oncol. 2011, 38, 473–483. [Google Scholar] [PubMed]
- Lee, Y.J.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Kim, K.J.; Lee, B.Y. Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Cypriani, B.; Limasset, B.; Carrié, M.L.; Le Doucen, C.; Roussie, M.; de Paulet, A.C.; Damon, M. Antioxidant activity of micronized diosmin on oxygen species from stimulated human neutrophils. Biochem. Pharmacol. 1993, 45, 1531–1535. [Google Scholar] [CrossRef]
- Liu, L.L.; Gong, L.K.; Wang, H.; Xiao, Y.; Wu, X.F.; Zhang, Y.H.; Xue, X.; Qi, X.M.; Ren, J. Baicalin inhibits macrophage activation by lipopolysaccharide and protects mice from endotoxin shock. Biochem. Pharmacol. 2008, 75, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-B.; Jun-Fang; Lu, X.Y.; Chen, X.J.; Chao-Tan; Sun, Z.L. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur. J. Pharmacol. 2009, 616, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Harasstani, O.A.; Moin, S.; Tham, C.L.; Liew, C.Y.; Ismail, N.; Rajajendram, R. Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm. Res. 2010, 59, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Nagasaki, Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J. Controll. Release 2015, 217, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Scicinski, J.; Oronsky, B.; Ning, S.; Knox, S.; Peehl, D.; Kim, M.M.; Langecker, P.; Fanger, G. NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cancio, M.; Fernández-Vitos, E.M.; Centelles, J.J.; Imperial, S. Sources of interference in the use of 2,3-diaminonaphthalene for the fluorimetric determination of nitric oxide synthase activity in biological samples. Clin. Chim. Acta 2001, 312, 205–212. [Google Scholar] [CrossRef]
- Tsikas, D. Circulating and excretory nitrite and nitrate: Their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide 2015, 45, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Girbes, A.R.J.; Beishuizen, A.; van Schijndel, R.J.M.S. Pharmacological treatment of sepsis. Fundam. Clin. Pharmacol. 2008, 22, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Lomas-Neira, J.; Chung, C.S.; Grutkoski, P.S.; Dunican, A.; Simms, H.H.; Cioffi, W.G.; Ayala, A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine 2005, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kubala, L.; Schmelzer, K.R.; Klinke, A.; Kolarova, H.; Baldus, S.; Hammock, B.D.; Eiserich, J.P. Modulation of arachidonic and linoleic acid metabolites in myeloperoxidase-deficient mice during acute inflammation. Free Radic. Biol. Med. 2010, 48, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-C.; Wang, Y.C.; Chien, Y.W.; Hou, Y.C.; Hu, Y.M.; Yeh, S.L. Effects of dietary fish oil supplementation on cellular adhesion molecule expression and tissue myeloperoxidase activity in hypercholesterolemic mice with sepsis. J. Nutr. Biochem. 2009, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Kubota, K.; Kita, J.; Shimoda, M.; Kato, M.; Mori, S.; Iso, Y.; Yamagishi, H.; Kojima, M. Aspartate aminotransferase-to-platelet ratio index is associated with liver cirrhosis in patients undergoing surgery for hepatocellular carcinoma. J. Surg. Res. 2015, 194, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Van Mierlo, K.M.C.; Schaap, F.G.; Dejong, C.H.; Olde Damink, S.W. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J. Hepatol. 2016, 65, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Khattab, H.; Fouad, A.; Hamza, M.; Mohey, M.A.; El-Akel, W.; Ghoneim, H.; Abul-Fotouh, A.; Esmat, G. Relation of ALT and AST levels to the histopathological changes in liver biopsies of patients with chronic hepatitis C genotype 4. Arab J. Gastroenterol. 2015, 16, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, L.M.; Ward, P.A. Mechanisms of inflammatory response syndrome in sepsis. Drug Discov. Today Dis. Mech. 2004, 1, 345–350. [Google Scholar] [CrossRef]
- Guo, T.L.; Chi, R.P.; Karrow, N.A.; Zhang, L.X.; Pruett, S.B.; Germolec, D.R.; White, K.L., Jr. Thalidomide enhances both primary and secondary host resistances to Listeria monocytogenes infection by a neutrophil-related mechanism in female B6C3F1 mice. Toxicol. Appl. Pharmacol. 2005, 209, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yan, W.; Liu, X.; Duan, M.; Zhang, X.; Xu, J. Effects of Hydroxyethyl Starch 130/0.4 on Pulmonary Capillary Leakage and Cytokines Production and NF-κB Activation in CLP-Induced Sepsis in Rats. J. Surg. Res. 2006, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, F.H.P.; Corrêa, P.B.; Oliveira-Pelegrin, G.R.; Rocha, M.J. Blocking systemic nitric oxide production alters neuronal activation in brain structures involved in cardiovascular regulation during polymicrobial sepsis. Neurosci. Lett. 2009, 453, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hillefass, L.M.; Griswold, D.E.; Brickson, B.; Albrightson-Winslow, C. Assessment of myeloperoxidase activity in whole rat kidney. J. Pharmacol. Methods 1990, 24, 285–295. [Google Scholar] [CrossRef]
- Devi, P.K.; Malar, S.D.; Nabavi, F.S.; Sureda, A.; Xiao, J.; Nabavi, M.S.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Auttachoat, W.; Zheng, J.F.; Chi, R.P.; Meng, A.; Guo, T.L. Differential surface expression of CD18 and CD44 by neutrophils in bone marrow and spleen contributed to the neutrophilia in thalidomide-treated female B6C3F1 mice. Toxicol. Appl. Pharmacol. 2007, 218, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S. Shock, inflammation and PARP. Pharmacol. Res. 2005, 52, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Walker, J.; Spur, B.; Rodriguez, A.; Yin, K. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot. Essent. Fatty Acids (PLEFA) 2015, 94, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B.; Hanpithakpong, W.; Tarning, J.; Anal, A.K. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind. Crops Prod. 2015, 77, 873–882. [Google Scholar] [CrossRef]
- Del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríguez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220. [Google Scholar] [CrossRef]
- Mahat, M.Y.A.; Kulkarni, N.M.; Vishwakarma, S.L.; Khan, F.R.; Thippeswamy, B.S.; Hebballi, V.; Adhyapak, A.A.; Benade, V.S.; Ashfaque, S.M.; Tubachi, S.; et al. Modulation of the cyclooxygenase pathway via inhibition of nitric oxide production contributes to the anti-inflammatory activity of kaempferol. Eur. J. Pharmacol. 2010, 642, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liaw, W.J.; Chen, T.H.; Lai, Z.Z.; Chen, S.J.; Chen, A.; Tzao, C.; Wu, J.Y.; Wu, C.C. Effects of a membrane-permeable radical scavenger, Tempol, on intraperitoneal sepsis-induced organ injury in rats. Shock 2005, 23, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.; Andrades, M.E.; Reinke, A.; Menna-Barreto, S.; Moreira, J.C.; Dal-Pizzol, F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit. Care Med. 2004, 32, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Kawata, J.; Yamamoto, T.; Ishimaru, Y.; Sakamoto, A.; Ono, T.; Narahara, S.; Sugiuchi, H.; Hirose, E.; Yamaguchi, Y. Mechanism of interferon-gamma production by monocytes stimulated with myeloperoxidase and neutrophil extracellular traps. Blood Cells Mol. Dis. 2015, 55, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tiyerili, V.; Camara, B.; Becher, M.U.; Schrickel, J.W.; Lütjohann, D.; Mollenhauer, M.; Baldus, S.; Nickenig, G.; Andrié, R.P. Neutrophil-Derived myeloperoxidase promotes atherogenesis and neointima formation in mice. Int. J. Cardiol. 2016, 204, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are commercially available.

| Absorbance Change/100 mg Liver Tissue ×100 | ||||
|---|---|---|---|---|
| 6 h | 24 h | |||
| Vehicle | 311 ± 40 | 266 ± 40 | ||
| Sham | 111 ± 30 | *** | 78 ± 40 | *** |
| Ch 5 mg/kg | 134 ± 30 | *** | 82 ± 20 | *** |
| Ka 1 mg/kg | 221 ± 20 | ** | 167 ± 60 | ** |
| Ka:Ch 3 mg/kg | 140 ± 10 | *** | 99 ± 20 | *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and Chrysin Synergies to Improve Septic Mice Survival. Molecules 2017, 22, 92. https://doi.org/10.3390/molecules22010092
Harasstani OA, Tham CL, Israf DA. Kaempferol and Chrysin Synergies to Improve Septic Mice Survival. Molecules. 2017; 22(1):92. https://doi.org/10.3390/molecules22010092
Chicago/Turabian StyleHarasstani, Omar A., Chau Ling Tham, and Daud A. Israf. 2017. "Kaempferol and Chrysin Synergies to Improve Septic Mice Survival" Molecules 22, no. 1: 92. https://doi.org/10.3390/molecules22010092
APA StyleHarasstani, O. A., Tham, C. L., & Israf, D. A. (2017). Kaempferol and Chrysin Synergies to Improve Septic Mice Survival. Molecules, 22(1), 92. https://doi.org/10.3390/molecules22010092







