Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant
Abstract
:1. Introduction
2. Cotton
2.1. Transgenic Cotton
3. Cotton Industry and Processing
3.1. Cotton Waste
4. Chemical Compounds in Cotton
4.1. Terpenes
4.1.1. Terpene Biosynthesis
4.1.2. Monoterpenes (C10)
4.1.3. Sesquiterpenes (C15)
4.1.4. Triterpenes (C30)
4.2. Phenols
4.2.1. Flavonoids
4.2.2. Phenolic Acids and Analogs
4.2.3. Tannins and Coumarins
4.3. Fatty Acids, Carbohydrates and Proteins
4.4. Variation in Cotton Chemical Composition
4.4.1. Genotypes and Varieties
4.4.2. Non-Transgenic and Cotton Transgenic Cotton Differences
5. Pharmacological Properties of Compounds in Cotton
5.1. Anti-Microbial Properties
5.2. Anti-Inflammatory and Anti-Oxidant Properties
5.3. Cytotoxic and Contraceptive Properties
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cotton Australia. The Cotton Plant. Available online: http://cottonaustralia.com.au/cotton-library/fact-sheets/cotton-fact-file-the-cotton-plant (accessed on 11 May 2015).
- Hegde, R.R.; Dahiya, A.; Kamath, M.G.; Gao, X.; Jangala, P.K. Cotton Fibres; Tickle college of Engineering, University of Tennessee: Knoxville, TN, USA, 2004. [Google Scholar]
- Raju, S.A.J.; Jonathan, H.K.; Rao, P.S. Traditional extraction of bark tannin from the mangrove tree Ceriops decantra (Griff.) Ding Hou and its use in treating cotton fishing nets. Nat. Prod. Radiance 2008, 7, 173–175. [Google Scholar]
- Cotton Australia. Uses of Cotton. Available online: http://cottonaustralia.com.au/australian-cotton/basics/uses-of-cotton (accessed on 11 May 2015).
- Blackwood, I. White Cottonseed—A Supplementary Feed for Beef Cattle; NSW DPI: Orange, New South Wales, Australia, 2007. [Google Scholar]
- Rogers, G.M.; Poore, M.H.; Paschal, J.C. Feeding cotton products to cattle. Verterinary Clin. N. Am. Food Anim. Pract. 2002, 18, 267–294. [Google Scholar] [CrossRef]
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [PubMed]
- Statistica World’s 10 Leading Cotton Producing Countries in 2013/2104 (in Metric Tons). Available online: http://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/ (accessed on 22 April 2015).
- Buser, M. Extruding Cotton Gin byproducts to reduce chemical residues. J. Cotton Sci. 2001, 5, 92–102. [Google Scholar]
- Knox, O.; Rochester, I.; Vadakattu, G.; Lawrence, L. Composting in Australian cotton production. Aust. Cotton Grow. 2006, 46–48. [Google Scholar]
- Stanley, A.W. Production and ginning of cotton. In Encyclopedia of Occupational Health and Safety, 89th ed.; Lee, I.A., Neefus, J.D., Stellman, J.M., Eds.; International Labor Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kennedy, J.B.; Rankins, D.L., Jr. Comparison of cotton gin trash and peanut hulls as low-cost roughage sources for growing beef cattle. Prof. Anim. Sci. 2008, 24, 40–46. [Google Scholar] [CrossRef]
- Wilde, C.; Johnson, J.; Farmer, M. Inventory of cotton gin trash on the Texas high plains and bio-energy feedstock potentials. Tex. J. Agric. Natl. Resour. 2010, 23, 42–49. [Google Scholar]
- Packham, R.; Royal, A.; Payne, C. Cottonseed meal in broiler diets. 1. The use of cottonseed meal as a replacement for soya bean meal in broiler starter diets. Aust. J. Exp. Agric. 1973, 13, 649–655. [Google Scholar] [CrossRef]
- Jeoh, T. Steam Explosion Pretreatment of Cotton Gin Waste for Fuel Ethanol Production. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1998. [Google Scholar]
- McIntosh, S.; Vancov, T.; Palmer, J.; Morris, S. Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour. Technol. 2014, 173, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Shivappa, R.; Chen, Y. Conversion of cotton wastes to bioenergy and value-added products. Am. Soc. Agric. Biol. Eng. 2008, 51, 2239–2246. [Google Scholar] [CrossRef]
- Shakhidoyatov, K.M.; Rashkes, A.M.; Khidyrova, N.K. Components of cottonplant leaves, their functional role and biological activity. Chem. Nat. Compd. 1997, 33, 605–616. [Google Scholar] [CrossRef]
- Bell, A.A. Physiology of secondary products. In Cotton Physiology; Mauney, J.R., Stewart, J.M., Eds.; The Cotton Foundation: Memphis, TN, USA, 1986; pp. 597–621. [Google Scholar]
- Hu, G.; Houston, N.L.; Pathak, D.; Schmidt, L.; Thelen, J.J.; Wendel, J.F. Genomically biased accumulation of seed storage proteins in allopolyploid cotton. Genetics 2011, 189, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.E.; Aboaba, S.O.; Ogunwande, I.A. Constituents and antimicrobial properties of the leaf essential oil of Gossypium barbadense (Linn.). J. Med. Plant Res. 2011, 5, 702–705. [Google Scholar]
- Perveen, S.S.; Qaisrani, T.M.; Bhutta, S.; Perveen, R.; Naqvi, S.H.M. HPLC analysis of cotton phenols and their contribution in bollworm resistance. J. Biol. Sci. 2001, 1, 587–590. [Google Scholar]
- Perveen, S.S.; Qaisrani, T.M.; Siddiqui, F.; Perveen, R.; Naqvi, S.H.M. Cotton plant volatiles and insect’s behavior. Pak. J. Biol. Sci. 2001, 4, 554–558. [Google Scholar]
- Iskhakov, N.I.; Sadykov, A.S.; Ismailov, A.I. The fatty acid composition of the phospholipids of cottonseed oil. Chem. Nat. Compd. 1965, 1, 152–154. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, B.A.; Aguilar, M.I.; King-Díaz, B.; Rivero, J.F.; Lotina-Hennsen, B. The sesquiterpenes β-caryophyllene and caryophyllene oxide isolated from Senecio salignus act as phytogrowth and photosynthesis inhibitors. Molecules 2012, 17, 1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Z.; Gao, Y.; Wang, Y. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014, 444, 451–454. [Google Scholar]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950. [Google Scholar] [CrossRef]
- Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E.; Rabinsky, T.; Rachmilevitch, S. Caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid. Based Complement. Altern. Med. 2012, 2012, 872394. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Converting waste agricultural biomass into a resource. In Compendium of Technologies; United Nations Environment Programme: Osaka/Shiga, Japan, 2009. [Google Scholar]
- Hossain, A.B.M.S.; Al-saif, A.M. Biodiesel fuel production from soybean oil waste as agricultural bio-resource. Aust. J. Crop Sci. 2010, 4, 538–542. [Google Scholar]
- Brentin, R.P. Soy-Based Chemicals and Materials: Growing the Value Chain. In ACS Symposiums; Amercian Chemical Soceity: Washington, DC, USA, 2014. [Google Scholar]
- Franco, H.C.J.; Pimenta, M.T.B.; Carvalho, J.L.N.; Magalhães, P.S.G.; Rossell, C.E.V.; Braunbeck, O.A.; Vitti, A.C.; Kölln, O.T.; Rossi Neto, J. Assessment of sugarcane trash for agronomic and energy purposes in Brazil. Sci. Agricola 2013, 70, 305–312. [Google Scholar] [CrossRef]
- Pode, R.; Diouf, B.; Pode, G. Sustainable rural electrification using rice husk biomass energy: A case study of Cambodia. Renew. Sust. Energy Rev. 2015, 44, 530–542. [Google Scholar] [CrossRef]
- Kuchelmeister, C.; Bauer, S. Rapid small-scale determination of extractives in biomass. Bioenergy Res. 2014, 8, 68–76. [Google Scholar] [CrossRef]
- Gotmare, V.; Singh, P.; Tule, B. Wild and cultivated species of Cotton. In Technical Bulletin; Central Institute for Cotton Research: Nagpur, India, 2000; Volume 5. [Google Scholar]
- Columbia Encyclopedia, cotton plant. In The Columbia Electronic Encyclopedia; Columbia University Press: New York, NY, USA, 2013.
- Liu, C.; Yuan, D.; Zhang, X.; Lin, Z. Isolation, characterization and mapping of genes differentially expressed during fibre development between Gossypium hirsutum and G. barbadense by cDNA-SRAP. J. Genet. 2013, 92, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Khaleequr, R.; Arshiya, S.; Shafeequr, R. Gossypium herbaceum Linn: An ethnological review. J. Pharm. Sci. Innov. 2012, 1, 1–5. [Google Scholar]
- Ayeni, M.J.; Oyeyemi, S.D.; Kayode, J.; Peter, G.P. Phytochemical, proximate and mineral analyses of the leaves of Gossypium hirsutum L. and Momordica charantia L. J. Nat. Sci. Res. 2015, 5, 99–107. [Google Scholar]
- Kazeem, M.I.; Abimbola, S.G.; Ashafa, A.O.T. Inhibitory potential of Gossypium arboreum leaf extracts on diabetes key enzymes, α-amylase and α-glucosidase. Bangladesh J. Pharmacol. 2013, 8. [Google Scholar] [CrossRef]
- Avci, U.; Pattathil, S.; Singh, B.; Brown, V.L.; Hahn, M.G.; Haigler, C.H. Cotton fiber cell walls of Gossypium hirsutum and Gossypium barbadense have differences related to loosely-bound xyloglucan. PLoS ONE 2013, 8, e56315. [Google Scholar] [CrossRef] [PubMed]
- Sawan, Z.M.; Hanna, L.I.; Gad El Karim, G.A.; McCuistion, W.L. Relationships between climatic factors and flower and boll production in Egyptian cotton (Gossypium barbadense). J. Arid Environ. 2002, 52, 499–516. [Google Scholar] [CrossRef]
- Gipson, J.R. Temperature effects on growth, development and fiber properties. In Cotton Physiology; Mauney, J.R., Stewart, J.M., Eds.; Cotton Foundation: Memphis, TN, USA, 1986; pp. 47–56. [Google Scholar]
- Wu, Z.; Soliman, K.M.; Zipf, A.; Saha, S.; Sharma, G.C.; Jenkins, J.N. Isolation and Characterization of Genes Differentially Expressed in Fiber of Gossypium barbadense L. J. Cotton Sci. 2005, 9, 166–174. [Google Scholar]
- Wendel, J.F.; Brubaker, C.L.; Seelanan, T. The origin and evolution of Gossypium. In Cotton Physiology; Mauney, J.R., Stewart, J.M., Eds.; Cotton Foundation: Memphis, TN, USA, 1986; pp. 1–18. [Google Scholar]
- Nandeshwar, S.B.; Moghe, S.; Chakrabarty, P.K.; Deshattiwar, M.K.; Kranthi, K.; Anandkumar, P.; Mayee, C.D.; Khadi, B.M. Agrobacterium-mediated transformation of Cry1Ac gene into shoot-tip meristem of diploid cotton Gossypium arboreum cv. RG8 and regeneration of transgenic plants. Plant Mol. Biol. Rep. 2009, 27, 549–557. [Google Scholar] [CrossRef]
- Momtaz, O.A.; Diab, A.A.; Madkour, M.A. Development of Transgenic Egyptian Cotton (Gossypium barbadense) Varieties from Meristematic Tissue. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 4–8 January 2000.
- Gomez, S.K.; Oosterhuis, D.M.; Hendrix, D.L.; Johnson, D.R.; Steinkraus, D.C. Diurnal pattern of aphid feeding and its effect on cotton leaf physiology. Environ. Exp. Bot. 2006, 55, 77–86. [Google Scholar] [CrossRef]
- Wilson, L. Cotton Aphids (Exotic Species). Available online: http://www.planthealthaustralia.com.au/wp-content/uploads/2013/01/Cotton-aphid-FS.pdf (accessed on 27 April 2015).
- Cook, D.R. Thrips Species Composition in Louisiana Cotton and Associated Management Strategies. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2003. [Google Scholar]
- Cook, D.R.; Leonard, R.B.; Burris, E.; Gore, J. Impact of thrips infesting cotton seedlings on cotton yield distribution and maturity. J. Cotton Sci. 2013, 17, 23–33. [Google Scholar]
- Stuart, R.R.; Gao, Y.-L.; Lei, Z.-R. Thrips: Pests of Concern to China and the United States. Agric. Sci. China 2011, 10, 867–892. [Google Scholar] [CrossRef]
- Badii, K.B.; Asante, S.K. Efficacy of some synthetic insecticides for control of cotton bollworms in northern Ghana. Afr. Crop Sci. J. 2012, 20, 59–66. [Google Scholar]
- Kranthi, S.; Dhawad, C.S.; Naidu, S.; Bharose, A.; Chaudhary, A.; Sangode, V.; Nehare, S.K.; Bajaj, S.R.; Kranthi, K.R. Susceptibility of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) to the Bacillus thuringiensis toxin Cry2Ab before and after the introduction of Bollgard-II. Crop Protect. 2009, 28, 371–375. [Google Scholar] [CrossRef]
- Zhang, B. Transgenic Cotton: Methods and Protocols; Humana Press: New York, NY, USA, 2013; p. 277. [Google Scholar]
- Krishna, V.V.; Qaim, M. Bt cotton and sustainability of pesticide reductions in India. Agric. Syst. 2012, 107, 47–55. [Google Scholar] [CrossRef]
- Perlak, F.J.; Oppenhuizen, M.; Gustafson, K.; Voth, R.; Sivasupramaniam, S.; Heering, D.; Carey, B.; Ihrig, R.A.; Roberts, J.K. Development and commercial use of Bollgard® cotton in the USA—Early promises versus today’s reality. Plant J. 2001, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kouser, S.; Qaim, M. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: A panel data analysis. Ecol. Econ. 2011, 70, 2105–2113. [Google Scholar] [CrossRef]
- Monsanto Cotton Research and Development. Available online: http://www.monsanto.com/global/au/products/pages/cotton-research-and-development.aspx (accessed on 27 April 2015).
- Ceeney, S.; Kauter, G.; Leven, T. World’s-Best Science: The basis of the Bollgard III RMP. Available online: http://www.cottongrower.com.au/images/articles/073f23c5c9b963000f807344a6115923.pdf (accessed on 28 April 2015).
- Maqbool, A.; Abbas, W.; Rao, A.Q.; Irfan, M.; Zahur, M.; Bakhsh, A.; Riazuddin, S.; Husnain, T. Gossypium arboreum GHSP26 enhances drought tolerance in Gossypium hirsutum. Biotechnol. Prog. 2010, 26, 21–25. [Google Scholar] [PubMed]
- Stewart, L.; Rossi, J. Using Cotton Byproducts in Beef Cattle Diets; University of Georgia: Athens, GA, USA, 2010. [Google Scholar]
- Cotton Australia. Processing, Exporting and Marketing. In Cotton Library; Cotton Australia: Sydney, NSW, Australia, 2015. [Google Scholar]
- Crossan, A.N.; Kennedy, I.R. Calculation of pesticide degradation in decaying cotton gin trash. Bull. Environ. Contam. Toxicol. 2008, 81, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Dı́az, M.J.; Madejón, E.; López, F.; López, R.; Cabrera, F. Composting of vinasse and cotton gin waste by using two different systems. Resour. Conserv. Recycl. 2002, 34, 235–248. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Crushed cotton gin compost on soil biological properties and rice yield. Eur. J. Agron. 2006, 25, 22–29. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vagena, A. Cotton gin trash compost in the substrate reduces the daminozide spray dose needed to produce compact potted chrysanthemum. Sci. Hort. 2012, 143, 102–108. [Google Scholar] [CrossRef]
- Duan, R.; Fedler, C.B.; Pearson, P.R. Environmental effects of using cotton burr compost mulch to establish roadside vegetation. Ecol. Eng. 2012, 39, 90–94. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.N.; Dowd, M.K.; Selling, G.W.; Biswas, A. Evaluation of cotton by-products as fillers for poly(lactic acid) and low density polyethylene. Ind. Crops Prod. 2012, 36, 127–134. [Google Scholar] [CrossRef]
- Plácido, J.; Capareda, S. Production of silicon compounds and fulvic acids from cotton wastes biochar using chemical depolymerization. Ind. Crops Prod. 2015, 67, 270–280. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Batz, S.; Trumbo, J. Composition and ethanol production potential of cotton gin residues. Appl. Biochem. Biotechnol. 2003, 105, 219–230. [Google Scholar] [CrossRef]
- Placido, J.; Imam, T.; Capareda, S. Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour. Technol. 2013, 139, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Yoav, B. Phenols in cotton seedlings resistant and susceptible to Alternaria macrospora. J. Phytopathol. 1986, 116, 1–10. [Google Scholar] [CrossRef]
- Thompson, A.C.; Baker, D.N.; Gueldner, R.C.; Hedin, P.A. Identification and quantitative analysis of the volatile substances emitted by maturing cotton in the field. Plant Physiol. 1971, 48, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.D.; Essenberg, M. (+)-δ-Cadinene is a product of sesquiterpene cyclase activity in cotton. Phytochemistry 1995, 39, 553–567. [Google Scholar] [CrossRef]
- Alchanati, I.; Patel, J.A.A.; Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Bell, A.A.; Cui, Y.; Magill, C.W. The enzymatic cyclization of nerolidyl diphosphate by δ-cadinene synthase from cotton stele tissue infected with Verticillium dahliae. Phytochemistry 1998, 47, 961–967. [Google Scholar] [CrossRef]
- Minyard, J.P.; Tumlinson, J.H.; Thompson, A.C.; Hedin, P.A. Constituents of the Cotton Bud. Sesquiterpene Hydrocarbons. J. Agric. Food Chem. 1966, 14, 332–336. [Google Scholar] [CrossRef]
- Elzen, G.W.; Williams, H.J.; Vinson, S.B. Isolation and identification of cotton synomones mediating searching behavior by parasitoidCampoletis sonorensis. J. Chem. Ecol. 1984, 10, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M. Medicinal Plants: Chemistry and Properties; Science Publishers: Enfield, NH, USA, 2006. [Google Scholar]
- Minyard, J.P.; Thompson, A.C.; Hedin, P.A. Constituents of the cotton bud. VIII. .beta.-Bisabolol, a new sesquiterpene alcohol. J. Org. Chem. 1968, 33, 909–911. [Google Scholar] [CrossRef]
- Hedin, P.A.; Thompson, A.C.; Gueldner, R.C.; Ruth, J.M. Isolation of bisabolene oxide from the cotton bud. Phytochemistry 1972, 11, 2118–2119. [Google Scholar] [CrossRef]
- McCormick, J.P.; Shinmyozu, T.; Pachlatko, J.P.; Schafer, T.R.; Gardner, J.W.; Stipanovic, R.D. Gossypium cadinanes and their analogs: Synthesis of lacinilene C, 2,7-dihydroxycadalene, and their methyl ethers. J. Org. Chem. 1984, 49, 34–40. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Puckhaber, L.S.; Reibenspies, J.H.; Williams, H.J. The absolute configuration of (−)-3-hydroxy-α-calacorene. Phytochemistry 2006, 67, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Davila-Huerta, G.; Hamada, H.; Davis, G.D.; Stipanovic, R.D.; Adams, C.M.; Essenberg, M. Cadinane-type sesquiterpenes induced in Gossypium cotyledons by bacterial inoculation. Phytochemistry 1995, 39, 531–536. [Google Scholar] [CrossRef]
- Zhang, H.L.; Nagatsu, A.; Okuyama, H.; Mizukami, H.; Sakakibara, J. Sesquiterpene glycosides from cotton oil cake. Phytochemistry 1998, 48, 665–668. [Google Scholar] [CrossRef]
- Williams, H.J.; Moyna, G.; Vinson, S.B.; Scott, A.I.; Bell, A.A.; Stipanovic, R.D. β-Caryophyllene derivatives from the wild cottons. Nat. Prod. Lett. 1997, 11, 25–30. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Greenblatt, G.A.; Beier, R.C.; Bell, A.A. 2-Hydroxy-7-methoxycadalene. The precursor of lacinilene C 7-methyl ether in Gossypium. Phytochemistry 1981, 20, 729–730. [Google Scholar] [CrossRef]
- Abraham, K.J.; Pierce, M.L.; Essenberg, M. The phytoalexins desoxyhemigossypol and hemigossypol are elicited by Xanthomonas in Gossypium cotyledons. Phytochemistry 1999, 52, 829–836. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Severino, L.; Luongo, D.; Rastrelli, L. Unusual cytotoxic sulfated cadinene-type sesquiterpene glycosides from cottonseed (Gossypium hirsutum). Tetrahedron 2008, 64, 5449–5453. [Google Scholar] [CrossRef] [Green Version]
- Stipanovic, R.D.; Bell, A.A.; O’Brien, D.H.; Lukefahr, M.J. Heliocide H1. A new insecticidal C25 terpenoid from cotton (Gossypium hirsutum). J. Agric. Food Chem. 1978, 26, 115–118. [Google Scholar] [CrossRef]
- Bell, A.A.; Stipanovic, R.D.; O’Brien, D.H.; Fryxell, P.A. Sesquiterpenoid aldehyde quinones and derivatives in pigment glands of Gossypium. Phytochemistry 1978, 17, 1297–1305. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Sato, D.; Awad, A.A.; Takeuchi, Y.; Yoneyama, K. Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants striga and orobanche, produced by cotton. Biosci. Biotechnol. Biochem. 2005, 69, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Stipanovic, R.D.; Bell, A.A.; O’Brien, D.H. Raimondal, a new sesquiterpenoid from pigment glands of Gossypium raimondii. Phytochemistry 1980, 19, 1735–1738. [Google Scholar] [CrossRef]
- Karimdzhanov, A.K.; Ismailov, A.I.; Abdullaev, Z.S.; Islambekov, S.Y.; Kamaev, F.G.; Sadykov, A.S. Structure of gossyvertin—A new phytoalexin of the cotton plant. Chem. Nat. Compd. 1976, 12, 211–214. [Google Scholar] [CrossRef]
- Bell, A.A.; Stipanovic, R.D.; Howell, C.R.; Fryxell, P.A. Antimicrobial terpenoids of Gossypium: Hemigossypol, 6-methoxyhemigossypol and 6-deoxyhemigossypol. Phytochemistry 1975, 14, 225–231. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Kim, H.L.; Altman, D.W.; Bell, A.A.; Kohel, R.J. Raimondalone, a sesquiterpene from a cotton interspecific hybrid. Phytochemistry 1994, 36, 953–956. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Bell, A.A.; Mace, M.E.; Howell, C.R. Antimicrobial terpenoids of Gossypium: 6-methoxygossypol and 6,6′-dimethoxygossypol. Phytochemistry 1975, 14, 1077–1081. [Google Scholar] [CrossRef]
- Nazarova, I.P.; Glushenkova, A.I.; Ul’chenko, N.T.; Moiseeva, G.P. Influence of wilt infection on the gossypol pigments of seeds and roots of a cotton plant of the variety Tashkent-1. Chem. Nat. Compd. 1989, 25, 54–57. [Google Scholar] [CrossRef]
- Pakudina, Z.P.; Rakhimov, A.A.; Sadykov, A.S. A chalcone from cotton plant flowers. Chem. Nat. Compd. 1969, 5, 109–110. [Google Scholar] [CrossRef]
- Hedin, P.A.; Jenkins, J.N.; Parrott, W.L. Evaluation of flavonoids in Gossypium arboreum (L.) cottons as potential source of resistance to tobacco budworm. J. Chem. Ecol. 1992, 18, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.A.; Howell, C.R.; Stipanovic, R.D. Cotton Host-microbe interactions. In Physiology of Cotton; Stewart, J.M., Oosterhuis, D., Heitholt, J.J., Mauney, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 187–205. [Google Scholar]
- Rangaswami, S.; Rao, P.S.; Seshadri, T.R. Pigments of cotton flowers. Proc. Indian Acad. Sci. (Math. Sci.) 1939, 9, 133–135. [Google Scholar]
- Yuan, S.; Yang, M.; Zhao, Y. A new flavonol glycoside from glandless cotton seeds. Acta Pharm. Sin. B 2012, 2, 42–45. [Google Scholar] [CrossRef]
- Elliger, C.A. Sexangularetin 3-glucoside-7-rhamnoside from Gossypium hirsutum. Phytochemistry 1984, 23, 1199–1201. [Google Scholar] [CrossRef]
- Wakelyn, P.J.; Stipanovic, R.D.; Bell, A.A. Identification of scopoletin in dried bract of the cotton plant. J. Agric. Food Chem. 1974, 22, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Tonn, W.H.; Schoch, E.P. Cotton Wax. Ind. Eng. Chem. 1946, 38, 413–415. [Google Scholar] [CrossRef]
- Rakhimkhanov, Z.B.; Karimdzhanov, A.K.; Ismailov, A.I.; Sadykov, A.S. A study of the anthocyanins of the cotton plant. Chem. Nat. Compd. 1968, 4, 164. [Google Scholar] [CrossRef]
- Jaquet, J.-P.; Buchala, A.; Meier, H. Changes in the non-structural carbohydrate content of cotton (Gossypium spp.) fibres at different stages of development. Planta 1982, 156, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, T.; Zhu, J. Carbohydrate profiles during cotton (Gossypium hirsutum L.) boll development and their relationships to boll characters. Field Crops Res. 2014, 164, 98–106. [Google Scholar] [CrossRef]
- Ryser, U.; Meier, H.; Holloway, P.J. Identification and localization of suberin in the cell walls of green cotton fibres (Gossypium hirsutum L. var. green lint). Protoplasma 1983, 117, 196–205. [Google Scholar] [CrossRef]
- Schmutz, A.; Buchala, A.J.; Ryser, U. Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol. 1996, 110, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; Turakhozhaev, M.T.; Shakirov, T.T. Isolation of raffinose from cottonseed meal. Chem. Nat. Compd. 1984, 20, 657–660. [Google Scholar] [CrossRef]
- Kuo, T.M.; VanMiddlesworth, J.F.; Wolf, W.J. Content of raffinose oligosaccharides and sucrose in various plant seeds. J. Agric. Food Chem. 1988, 36, 32–36. [Google Scholar] [CrossRef]
- Randall, D.D.; Tolbert, N.E.; Gremel, D. 3-Phosphoglycerate phosphatase in plants: II. Distribution, physiological considerations, and comparison with P-glycolate phosphatase. Plant Physiol. 1971, 48, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.S.; Gilbert, R.D.; Fornes, R.E. Isolation of indole-3 carboxaldehyde from cotton plant parts. Text. Res. J. 1983, 53, 51–53. [Google Scholar] [CrossRef]
- Hong, Y.K.; Gilbert, R.D.; Fornes, R.E. Quantitative determination of indole-3 carboxyaldehyde in aqueous extracts of cotton bracts by HPLC. Text. Res. J. 1985, 55, 17–19. [Google Scholar] [CrossRef]
- Hedin, P.A.; Thompson, A.C.; Gueldner, R.C. Constituents of cotton bud essential oil. Phytochemistry 1975, 14, 2087–2088. [Google Scholar] [CrossRef]
- Hanny, B.W.; Gueldner, R.C. Surface lipids of the glabrous cotton (Gossypium hirsutum) strain, Bayou SM1. J. Agric. Food Chem. 1976, 24, 401–403. [Google Scholar] [CrossRef]
- Opitz, S.; Kunert, G.; Gershenzon, J. Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 2008, 34, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, M.C.; Mazzafera, P. A genomic approach to characterization of the Citrus terpene synthase gene family. Genet. Mol. Biol. 2007, 30, 832–840. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.W.; Tumlinson, J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Rose, U.S.R.; Tumlinson, J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 2005, 222, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Manukian, A.; Heath, R.R.; Turlings, T.C.; Tumlinson, J.H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. USA 1994, 91, 11836–11840. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Q.; Wu, X.-M.; Ruan, J.-X.; Hu, W.-L.; Mao, Y.-B.; Chen, X.-Y.; Wang, L.-J. Isolation and characterization of terpene synthases in cotton (Gossypium hirsutum). Phytochemistry 2013, 96, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.V.; Nagasampagi, B.A.; Sivakumar, M. Chemistry of Natural Products; Narosa publishing house: Delhi, India, 2005. [Google Scholar]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008. [Google Scholar]
- Sallaud, C.; Rontein, D.; Onillon, S.; Jabès, F.; Duffé, P.; Giacalone, C.; Thoraval, S.; Escoffier, C.; Herbette, G.; Leonhardt, N.; et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell 2009, 21, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; Souza, D.P.D.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [PubMed]
- Banthorpe, D.V.; Charlwood, B.V.; Francis, M.J.O. Biosynthesis of monoterpenes. Chem. Rev. 1972, 72, 115–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Q.; Ruan, J.-X.; Wang, L.-J.; Chen, X.-Y. Isolation and characterization of volatile terpene synthases in cotton. In Proceedings of the Plant and Animal Genome XXII, San Diego, CA, USA, 11–15 January 2014.
- Opitz, S.; Nes, W.D.; Gershenzon, J. Both methylerythritol phosphate and mevalonate pathways contribute to biosynthesis of each of the major isoprenoid classes in young cotton seedlings. Phytochemistry 2014, 98, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Machado Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Legé, K.E. Tannins in Cotton. In Cotton; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 42, pp. 350–363. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Bressler, R.; Wakil, S.J. Studies on the mechanism of fatty acid synthesis: IX. The conversion of malonyl coenzyme a to long chain fatty acids. J. Biol. Chem. 1961, 236, 1643–1651. [Google Scholar]
- Dowd, M.K.; Boykin, D.L.; Meredith, W.R., Jr.; Campbell, B.T.; Bourland, F.M.; Gannaway, J.R.; Glass, K.M.; Zhang, J. Fatty acid profiles of cottonseed genotypes from the national cotton variety trials. J. Cotton Sci. 2010, 14, 64–73. [Google Scholar]
- King, E.E.; Leffler, H.R. Nature and patterns of proteins during cotton seed development. Plant Physiol. 1979, 63, 260–263. [Google Scholar] [CrossRef] [PubMed]
- John, M.E.; Keller, C. Characterization of mRNA for a proline-rich protein of cotton fiber. Plant Physiol. 1995, 108, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-L.; Zhang, D.-J.; Wu, Y.-F.; Qin, L.-X.; Huang, G.-Q.; Li, J.; Li, L.; Li, X.-B. Cotton PRP5 gene encoding a proline-rich protein is involved in fiber development. Plant Mol. Biol. 2013, 82, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Idowu, O.J.; Wedegaertner, T.; Hughs, S.E. Genetic variation and comparative analysis of thrips resistance in glandless and glanded cotton under field conditions. Euphytica 2014, 199, 373–383. [Google Scholar] [CrossRef]
- McMichael, S.C. Hopi cotton, a source of cotton-seed free of gossypol pigments. Agron. J. 1959, 51, 630. [Google Scholar] [CrossRef]
- Lusas, E.W.; Jividen, G.M. Glandless cottonseed: A review of the first 25 years of processing and utilization research. J. Am. Oil Chem. Soc. 1987, 64, 839–854. [Google Scholar] [CrossRef]
- Romano, G.B.; Scheffler, J.A. Lowering seed gossypol content in glanded cotton (Gossypium hirsutum L.) lines. Plant Breed. 2008, 127, 619–624. [Google Scholar] [CrossRef]
- Lawhon, J.T.; Cater, C.M.; Mattil, K.F. Evaluation of the food potential of sixteen varieties of cottonseed. J. Am. Oil Chem. Soc. 1977, 54, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Nergiz, C.; Yalçin, H.; Yildiz, H. Some analytical characters of cottonseed varieties grown in Turkey. Grasasy Aceites 1997, 48, 411–414. [Google Scholar] [CrossRef]
- Downes, S.; Mahon, R. Evolution, ecology and management of resistance in Helicoverpa spp. to Bt cotton in Australia. J. Invertebr. Pathol. 2012, 110, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Héma, O.; Somé, H.N.; Traoré, O.; Greenplate, J.; Abdennadher, M. Efficacy of transgenic cotton plant containing the Cry1Ac and Cry2Ab genes of Bacillus thuringiensis against Helicoverpa armigera and Syllepte derogata in cotton cultivation in Burkina Faso. Crop Protect. 2009, 28, 205–214. [Google Scholar] [CrossRef]
- Yan, F.; Xu, C.; Bengtsson, M.; Witzgall, P.P. Volatile compositions of transgenic Bt cotton and their electrophysiological effects on the cotton bollworm. Kun Chong Xue Bao 2002, 45, 425–429. [Google Scholar]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.-K. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Wanzala, W.; Hassanali, A.; Mukabana, W.R.; Takken, W. Repellent activities of essential oils of some plants used traditionally to control the brown ear tick, Rhipicephalus appendiculatus. J. Parasitol. Res. 2014, 2014, 434506. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, R.K.; Singhal, K.K.; Ebrahimi, S.H.; Rajput, Y.S.; Mohini, M. Comparative nutritional evaluation of transgenic cottonseeds containing Cry1C protein for ruminant feeding. Livest. Res. Rural Dev. 2011, 23, 14. [Google Scholar]
- Khan, V.; Najmi, A.K.; Akhtar, M.; Aqil, M.; Mujeeb, M.; Pillai, K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied Sci. 2012, 4, 27–42. [Google Scholar] [PubMed]
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [PubMed]
- Maganha, E.G.; Halmenschlager, R.D.C.; Rosa, R.M.; Henriques, J.A.P.; Ramos, A.L.L.D.P.; Saffi, J. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 2010, 118, 1–10. [Google Scholar] [CrossRef]
- Olorunnisola, S.K.; Asiyanbi, H.T.; Hammed, A.M.; Simsek, S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014, 21, 455–462. [Google Scholar]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; Lima, E.D.O.; de Souza, E.L.; Diniz, M.D.F.M.; Trajano, V.N.; de Medeiros, I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Moon, J.-H.; Seong, K.-Y.; Park, K.-H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Mathew, L.; Vijayalakshmi, N.R.; Helen, A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Çelİk, K.; Toğar, B.; Türkez, H.; TaŞpinar, N. In vitro cytotoxic, genotoxic, and oxidative effects of acyclic sesquiterpene farnesene. Turk. J. Biol. 2014, 38, 253–259. [Google Scholar] [CrossRef]
- Rozza, A.L.; Moraes Tde, M.; Kushima, H.; Tanimoto, A.; Marques, M.O.M.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; García, M.; Steinbauer, S.; Setzer, W.N.; Scull, R.; Gille, L.; Monzote, L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015, 145, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jia, Y.; Lee, J.H.; Jun, H.-J.; Lee, H.-S.; Hwang, K.-Y.; Lee, S.-J. trans-Caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-α. Bioorg. Med. Chem. Lett. 2014, 24, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Jacob, S.E. Bisabolol. Dermatitis 2010, 21, 57–58. [Google Scholar] [PubMed]
- Darra, E.; Abdel-Azeim, S.; Manara, A.; Shoji, K.; Maréchal, J.-D.; Mariotto, S.; Cavalieri, E.; Perbellini, L.; Pizza, C.; Perahia, D.; et al. Insight into the apoptosis-inducing action of α-bisabolol towards malignant tumor cells: Involvement of lipid rafts and Bid. Arch. Biochem. Biophys. 2008, 476, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.-C.; Lee, D.-S.; Ahn, K.S.; Cho, S.K. Cytotoxic activity of beta-caryophyllene oxide isolated from jeju guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- Essenberg, M.; Grover, P.B.; Cover, E.C. Accumulation of antibacterial sesquiterpenoids in bacterially inoculated Gossypium leaves and cotyledons. Phytochemistry 1990, 29, 3107–3113. [Google Scholar] [CrossRef]
- Humphrey, A.J.; Beale, M.H. Strigol: Biogenesis and physiological activity. Phytochemistry 2006, 67, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, N.; Sugimoto, Y.; Kato, M.; Inanaga, S.; Yoneyama, K. (+)-Strigol, a witchweed seed germination stimulant, from Menispermum dauricum root culture. Phytochemistry 2003, 62, 1115–1119. [Google Scholar] [CrossRef]
- Khaledi, H.; Alhadi, A.A.; Yehye, W.A.; Ali, H.M.; Abdulla, M.A.; Hassandarvish, P. Antioxidant, cytotoxic activities, and structure–activity relationship of gallic acid-based indole derivatives. Arch. Pharm. 2011, 344, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.M. Gossypol: A contraceptive for men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef]
- Randel, R.D.; Chase, C.C.; Wyse, S.J. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci. 1992, 70, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Wungsintaweekul, B.; Umehara, K.; Miyase, T.; Noguchi, H. Estrogenic and anti-estrogenic compounds from the Thai medicinal plant, Smilax corbularia (Smilacaceae). Phytochemistry 2011, 72, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, D.; Batista, J.M.; Rodrigues, J.; Benfatti, A.C.; Rodrigues, C.M.; Dos Santos, L.C.; Furlan, M.; Vilegas, W. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality 2010, 22, 726–733. [Google Scholar] [PubMed]
- Razavi, S.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. J. Bioorg. Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Sermakkani, M.; Thangapandian, V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Lara-Hernández, B.; Hernández-León, A.; Villafuerte-Robles, L. Effect of stearic acid on the properties of metronidazole/methocel K4M floating matrices. Braz. J. Pharm. Sci. 2009, 45, 497–505. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [PubMed]
- Tarmadi, D.; Himmi, S.K.; Yusuf, S. The efficacy of the oleic acid isolated from Cerbera manghas L. seed against a subterranean termite, Coptotermes gestroi wasmann and a drywood termite, Cryptotermes cynocephalus Light. Procedia Environ. Sci. 2014, 20, 772–777. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. 2007, 45, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Viernes, L.B.G.; Garcia, R.N.; Torio, M.A.O.; Angelia, M.R.N. Antihypertensive peptides from vicilin, the major storage protein of mung bean (Vigna radiata (L.) R. Wilczek). J. Biol. Sci. 2012, 12, 393–399. [Google Scholar]















| Compounds | Molecular Formula | Molecular Weight (g/mol) | References |
|---|---|---|---|
| Terpenes | |||
| Monoterpenes | C10H16 | 136.24 | |
| Camphene | [21] | ||
| Limonene | [21,23] | ||
| Myrcene | [23] | ||
| Ocimene | [21,23] | ||
| α-pinene | [23] | ||
| β-pinene | [23] | ||
| Sabinene | [21,23] | ||
| α-Thujene | [21] | ||
| Sesquiterpenes | C15H24 | 204.35 | |
| α-Bergamotene | [78] | ||
| Bisabolene | [78] | ||
| 1(10),4-Cadinadiene; (6β,7β)-form | [79,80] | ||
| Caryophyllene | [78] | ||
| Copaene | [81] | ||
| Guaiadiene: (4β,5α,7β)-form | [81] | ||
| Farnesene | [78] | ||
| Humulene | [81,82] | ||
| Terpene derivatives | |||
| α and β-Amyrin | C30H50O | 426.72 | [83] |
| Bisabolol | C15H26O | 222.37 | [82,84] |
| 1,3,5,10-Bisabolatetraen-7-ol | C15H22O | 218.34 | [82] |
| Bisabolene oxide | C15H24O | 220.35 | [85] |
| 1(10),4-Cadinadien-2-ol; (2ξ,6β,7β)-form | C15H24O | 220.35 | [86] |
| 1,3,5,7,9-Cadinapentaene-3,9-diol | C15H18O2 | 230.31 | [86] |
| 1,3,5,7,9-Cadinapentaene-3,9-diol; 3-Me ether | C16H20O2 | 244.33 | [86] |
| 1,3,5,9-Cadinatetraene; 7αH-form, 3-Hydroxy | C15H20O | 216.32 | [87] |
| 1,3,5-Cadinatriene-3,9-diol; (7α,9α,10α)-form, 9-Ketone | C15H20O2 | 232.32 | [88] |
| 1,3,5-Cadinatriene-3,9-diol; (7β,10α)-form, 9-Ketone | C15H20O2 | 232.32 | [88] |
| 1,3,5-Cadinatriene-3,9,10-triol; (7β,9β,10α)-form, 9-O-β-d-Glucopyranoside | C21H32O8 | 412.48 | [89] |
| 3(15),6-Caryophylladien-12-ol; (6E)-form | C15H24O | 220.35 | [90] |
| 3(15),6-Caryophylladien-12-ol; (6E)-form, 6α,7β-Epoxide, Ac | C17H26O3 | 278.39 | [90] |
| Caryophyllene oxide | C15H24O | 220.35 | [82] |
| 3,10-Dihydroxy-1,3,5,7-cadinatetraen-9-one | C15H18O3 | 246.31 | [91] |
| 8,9-Dihydroxy-2,5-dioxo-1(6),3,7,9-cadinatetraen-14-al | C15H14O5 | 274.27 | [91] |
| 2,14-Epoxy-1,3,5,7,9-cadinapentaene-8,9-diol | C15H16O3 | 244.29 | [92] |
| 2,14-Epoxy-1,3,5,7,9-cadinapentaene-8,9,12-triol; 15-Hydroxy, 9-O-(6-O-sulfo-β-d-glucopyranoside) | C21H26O13S | 518.50 | [93] |
| Heliocide H1 | C25H30O5 | 410.51 | [93,94] |
| Heliocide H1; 7-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H2 | C25H30O5 | 410.51 | [93] |
| Heliocide H2; 3-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H3 | C25H30O5 | 410.51 | [93] |
| Heliocide H3; 3-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H4 | C25H30O5 | 410.51 | [93] |
| Heliocide H4; 3-Me ether | C26H32O5 | 424.54 | [95] |
| β-Sitosterol | C29H50O | 414.71 | [83] |
| Strigol | C19H22O6 | 346.38 | [96,97] |
| 2,3,8,9-Tetrahydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | C16H18O5 | 290.32 | [98] |
| 2,3,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | C16H18O4 | 274.32 | [99] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al | C15H16O4 | 260.29 | [100] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-Deoxy | C15H16O3 | 244.29 | [100] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-Me ether | C16H18O4 | 274.32 | [100] |
| 3,8,9-Trihydroxy-2,5-dioxo-1(6),3,7,9-cadinatetraen-14-al; 3-Me ether | C16H16O6 | 304.30 | [101] |
| Phytol | C20H40O | 296.54 | [18] |
| Phenols | |||
| Phenolic acids | |||
| Benzoic acid | C7H6O2 | 122.12 | [22] |
| Chlorogenic acid | C16H18O9 | 354.31 | [22] |
| Ferrulic acid | C10H10O4 | 194.18 | [22] |
| Gallic acid | C7H6O5 | 170.12 | [22] |
| Gentisic acid | C7H6O4 | 154.12 | [22] |
| P-coumaric acid | C9H8O3 | 164.16 | [22] |
| 4-hydroxybenzoic acid | C7H6O3 | 138.12 | [22] |
| 3,4-Dihydroxybenzoic acid | C7H6O4 | 154.12 | [22] |
| Syringic acid | C9H10O5 | 198.17 | [22] |
| Phenolic acid analogs | |||
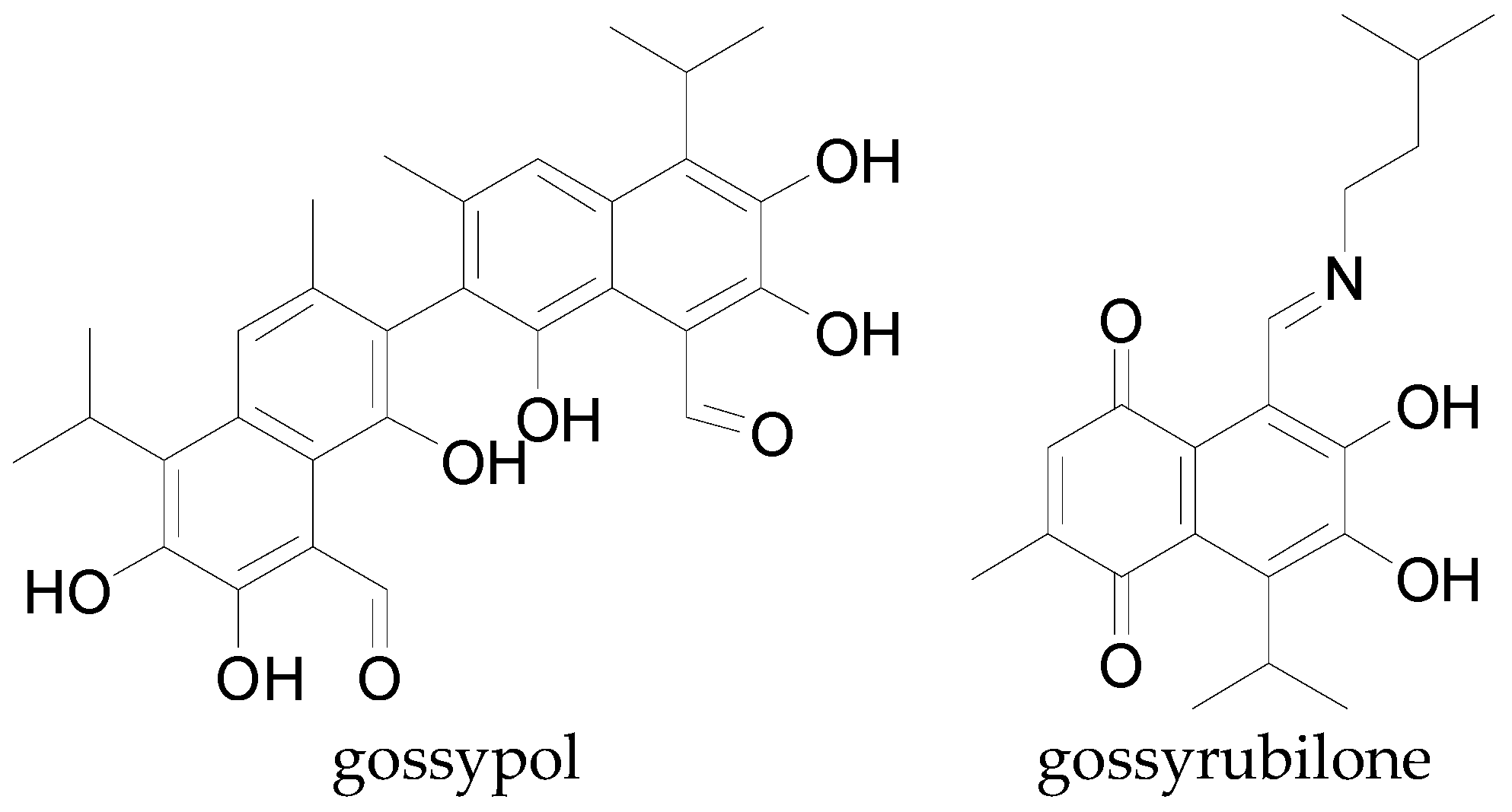
| Gossypol; (±)-form, 6-Me ether | C31H32O8 | 532.59 | [102] |
| Gossypol; (±)-form, 6,6′-di-Me ether | C32H34O8 | 546.62 | [102] |
| Gossypol; (+)-form | C30H30O8 | 518.56 | [83] |
| Gossypurpurin | C60H56N2O13 | 1013.11 | [103] |
| Gossyrubilone | C20H25NO4 | 343.42 | [95] |
| Flavonoids | |||
| α-2′,3,3′,4,4′,6-Heptahydroxychalcone; 2′-O-d-Glucopyranoside | C12H22O13 | 482.40 | [104] |
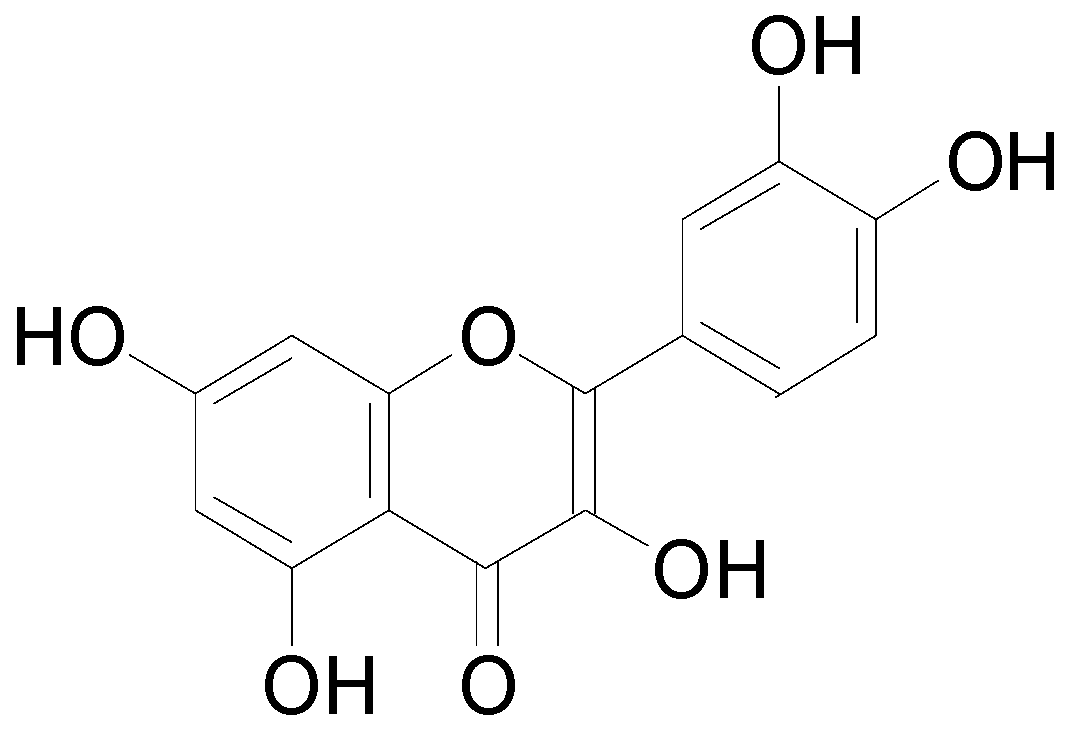
| 3,3′,4′,5,7,8-Hexahydroxyflavone; 3-O-β-d-Glucopyranoside | C21H20O13 | 480.38 | [19] |
| Gossypetin 7-glucoside | C21H20O13 | 480.38 | [19] |
| 3,3′,4′,5,7,8-Hexahydroxyflavone; 8-O-α-l-Rhamnopyranoside | C21H20O12 | 464.38 | [105] |
| Kaempferol 3-glycosides; Monoglycosides, 3-O-α-d-Glucopyranoside | C21H20O11 | 448.38 | [19] |
| 3,3′,4′,5,7-Pentahydroxyflavan; (2S,3R)-form | C15H14O6 | 290.27 | [106] |
| 3,3′,4′,5,7-Pentahydroxyflavone; 3′-O-β-d-Glucopyranoside | C21H20O12 | 464.38 | [105] |
| 3,4′,5,7,8-Pentahydroxyflavone | C15H10O7 | 302.24 | [107] |
| Quercetin 3-glycosides; Disaccharides, 3-O-[β-d-Galactopyranosyl-(1→6)-β-d-glucopyranoside] | C27H30O17 | 626.52 | [105] |
| Quercetin 3-glycosides; Tetra- and higher saccharides, 3-O-[α-d-Apiofuranosyl-(1→5)-β-d-apiofuranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranoside] | C37H46O24 | 874.76 | [108] |
| 3,3′,5,7-Tetrahydroxy-4′-methoxyflavone | C16H12O7 | 316.27 | [19] |
| 3,4′,5,7-Tetrahydroxy-8-methoxyflavone; 3-O-β-d-Glucopyranoside, 7-O-α-l-rhamnopyranoside | C28H32O16 | 624.55 | [109] |
| Other Phenols | |||
| Scopoletin | C10H8O4 | 192.17 | [110] |
| Fatty acids and Lipids | |||
| 11,14-Eicosadienoic acid | C20H36O2 | 308.50 | [24] |
| Hexadecanoic acid | C16H32O2 | 256.43 | [24] |
| 9-Hexadecanoic acid; (Z)-form | C16H30O2 | 254.41 | |
| Octadecanoic acid | C18H36O2 | 284.48 | [24] |
| 9-Octadecenoic acid; (Z)-form | C18H34O2 | 282.47 | [24] |
| 9,12-Octadecadienoic acid; (Z,Z)-form | C18H32O2 | 280.45 | [18] |
| 9,12,15-Octadecatrienoic acid; (Z,Z,Z)-form | C18H30O2 | 278.43 | [18] |
| Tetradecanoic acid (myristic acid) | C14H28O2 | 228.37 | [24] |
| Triacontanoic acid | C30H60O2 | 452.80 | [111] |
| Carbohydrates | |||
| Cellulose | C6H10O5 | 162.14 | [25] |
| Cyanidin 3-glycosides; Disaccharides, 3-O-[β-d-Xylopyranosyl-(1→4)-β-d-glucopyranoside] | C26H29O15 | 581.51 | [112] |
| 6-O-α-d-Galactopyranosyl-d-glucose | C12H22O11 | 342.30 | [113,114] |
| Glycerol 1-alkanoates; Glycerol 1-(22-hydroxydocosanoate), 22′-O-(3,4-Dihydroxycinnamoyl) | C34H56O8 | 592.81 | [115,116] |
| Raffinose | C18H32O16 | 504.44 | [117,118] |
| Proteins | |||
| 3-Phosphoglycerate phosphatase | [119] | ||
| Vicilin A and B | [20] | ||
| Legumin Aand B | [20] | ||
| Hydrocarbons | |||
| 1H-Indole-3-carboxaldehyde | C12H7NO | 145.16 | [120,121] |
| 1-Methyl-2-propylbenzene | C10H14 | 134.22 | [122] |
| Octatriacontane | C38H78 | 535.03 | [123] |
| Alcohols | |||
| Dotriacontanol | C32H66O | 466.88 | [18] |
| 1-Tetratriacontanol | C34H70O | 494.93 | [18] |
| Triacontanol | C30H62O | 438.81 | [18] |
| Compounds | Biological Activity | References |
|---|---|---|
| Terpenes | ||
| camphene | Aromatic properties, antioxidants effects | [21] |
| limonene | Flavouring properties. gastro-protective effects, anti-cancer and anti-inflammatory activity | [28,142] |
| myrcene | Analgesic effects, anti-microbial activity, anti-inflammatory activity, anti-catabolic activity | [171,172] |
| α and β-pinene | Gastro-protective effects, anti- microbial and ant-inflammatory effects | [28,174,180] |
| sabinene | Anti-microbial activity, anti-oxidant activity | [21] |
| α-thujene | Pungent activity | [21] |
| caryophyllene | Ant-inflammatory effects, anti-microbial activity, regulation of cellular lipid metabolism, flavouring properties | [27,32,181,182] |
| farnesene | Anti-oxidant effects | [179] |
| humulene | Anti-inflammatory properties, aromatic properties and cytotoxic activity | [29,32] |
| bisabolol | Aromatic properties, anti-inflammatory effects, anti-carcinogenic activity, anti-microbial and anti-oxidative properties | [28,183,184] |
| caryophyllene oxide | Cytotoxic activity, phytogrowth inhibition, analgesic and anti-inflammatory activity | [177] [26,185] |
| 3,10-dihydroxy-1,3,5,7-cadinatetraen-9-one | Phytoalexin, antifungal agent | [92,186] |
| β-sitosterol | Antimicrobial activity, anti-hypercholesteraemic and anti-inflammatory activity | [18,28] |
| strigol | Germination stimulant | [96,187,188] |
| 2,3,9-trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | Phytoalexin | [99] |
| 2,8,9-trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-deoxy | Antifungal activity | [92] |
| Phenols | ||
| chlorogenic acid | Anti-oxidant and anti-mutagenic activity | [144] |
| gallic acid | Antioxidant activity, cytotoxic activity | [189,190] |
| 4-hydroxybenzoic acid | Anti-microbial activity, used as preservative, oestrogenic activity, anti-inflammatory and anti-oxidant activity | [175,176,191] |
| gossypol; (+)-form | Contraceptive and hypokalemic activity | [30,192,193] |
| 3,3′,4′,5,7-pentahydroxyflavan; (2S,3R)-form | Cytotoxic and phytotoxic activity | [194,195] |
| 3,3′,4′,5,7-pentahydroxyflavone; 3′-O-β-d-glucopyranoside | Enzyme inhibitor, cytotoxic, anti-oxidant activity | [196] |
| scopoletin | Anti-spasmodic and anti-inflammatory activity | [19] |
| Fatty acids | ||
| 11,14-eicosadienoic acid | Hormonal activity | [18] |
| hexadecanoic acid | Anti-microbial and anti-inflammatory activity | [197] |
| octadecanoic acid | Pharmaceutical excipient, surfactant and softening activity | [198] |
| 9-octadecenoic acid; (Z)-form | Insecticidal, anti-bacterial and fungicidal activity | [199,200,201] |
| tetradecanoic acid | Defoaming agent, flavour adjuvant used in food processing | [202] |
| Carbohydrates | ||
| cellulose | Capsule and tablet diluent | [203] |
| Proteins | ||
| 3-phosphoglycerate phosphatase | Enzyme activity | [119] |
| vicilin | Anti-hypertensive activity | [204] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbuta, M.A.; McIntosh, S.; Waters, D.L.E.; Vancov, T.; Liu, L. Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant. Molecules 2017, 22, 93. https://doi.org/10.3390/molecules22010093
Egbuta MA, McIntosh S, Waters DLE, Vancov T, Liu L. Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant. Molecules. 2017; 22(1):93. https://doi.org/10.3390/molecules22010093
Chicago/Turabian StyleEgbuta, Mary A., Shane McIntosh, Daniel L. E. Waters, Tony Vancov, and Lei Liu. 2017. "Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant" Molecules 22, no. 1: 93. https://doi.org/10.3390/molecules22010093






