Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro
Abstract
:1. Introduction
2. Results
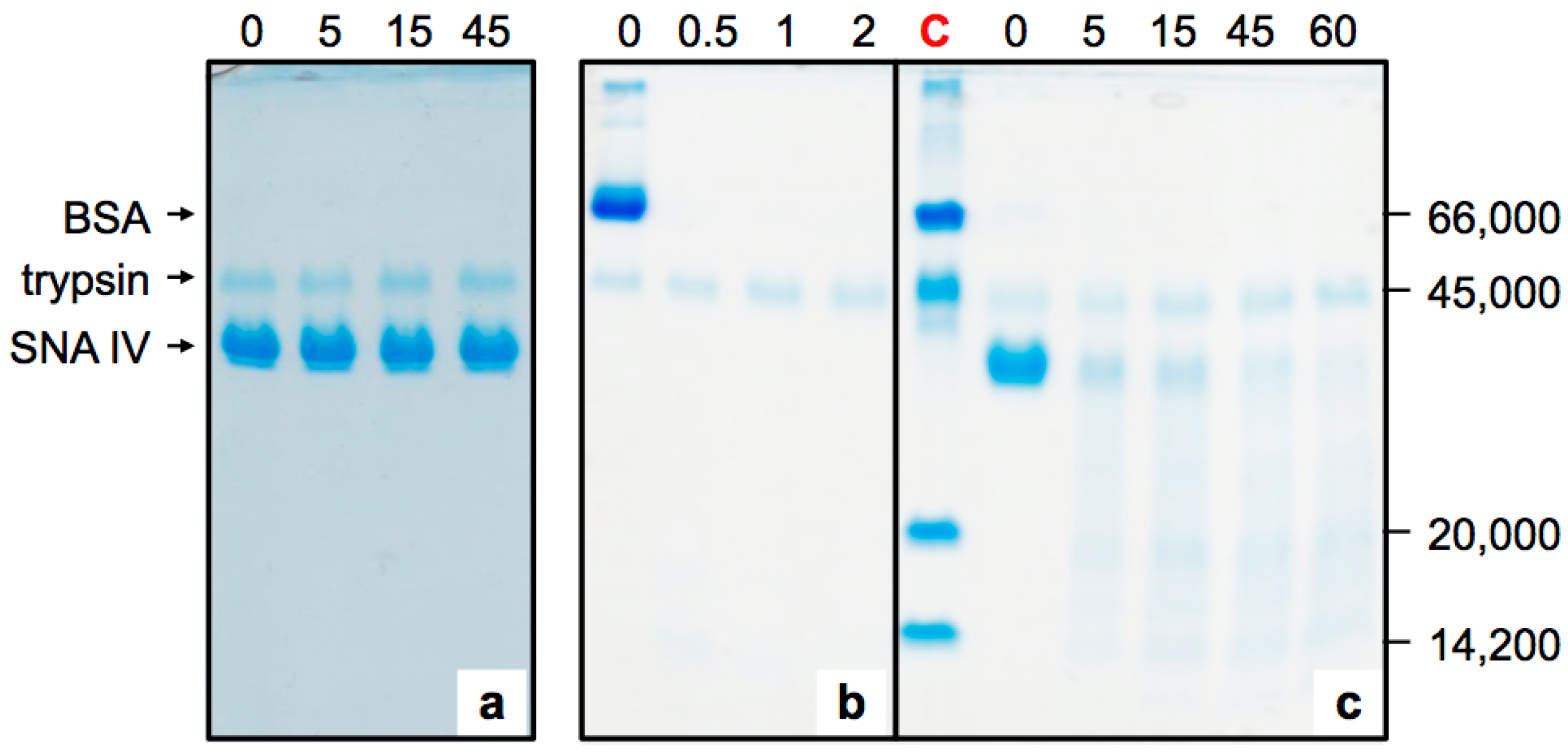
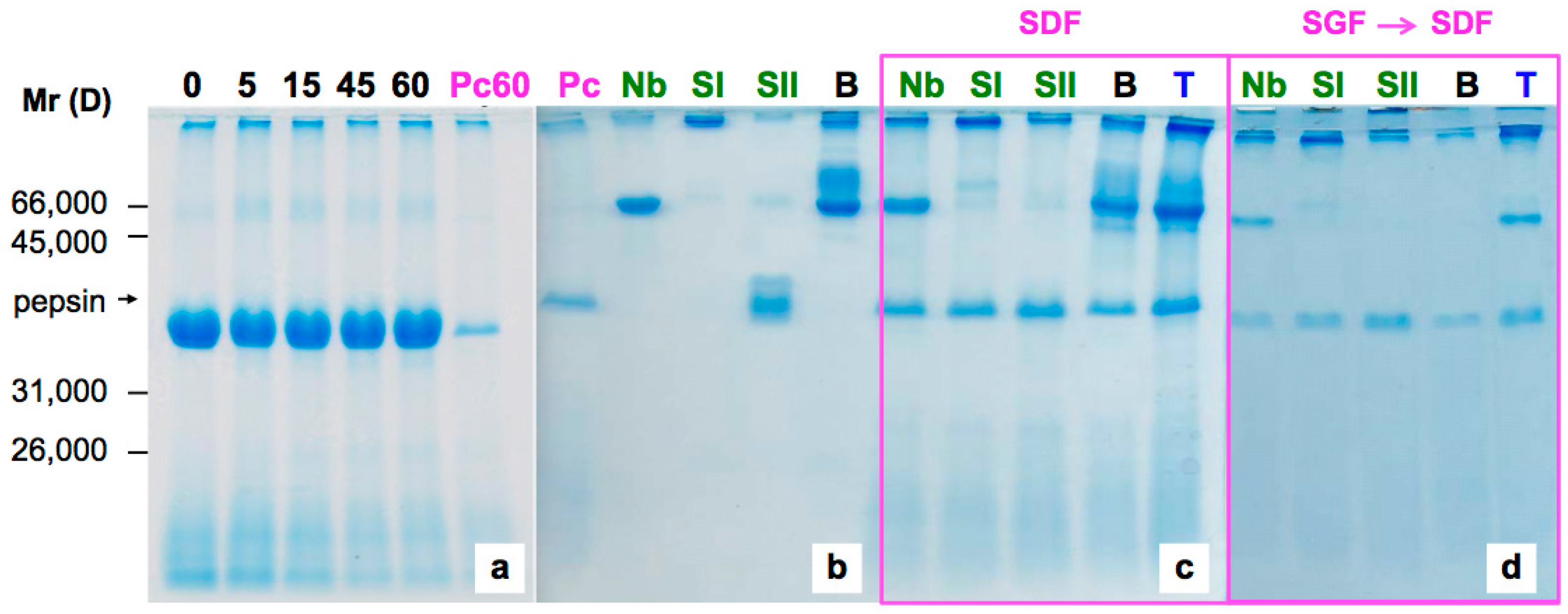
2.1. Sensitivity of Elderberry Purified Lectins to Simulated Gastric Fluid
2.2. Sensitivity of Elderberry Lectins to Simulated Duodenal Fluid
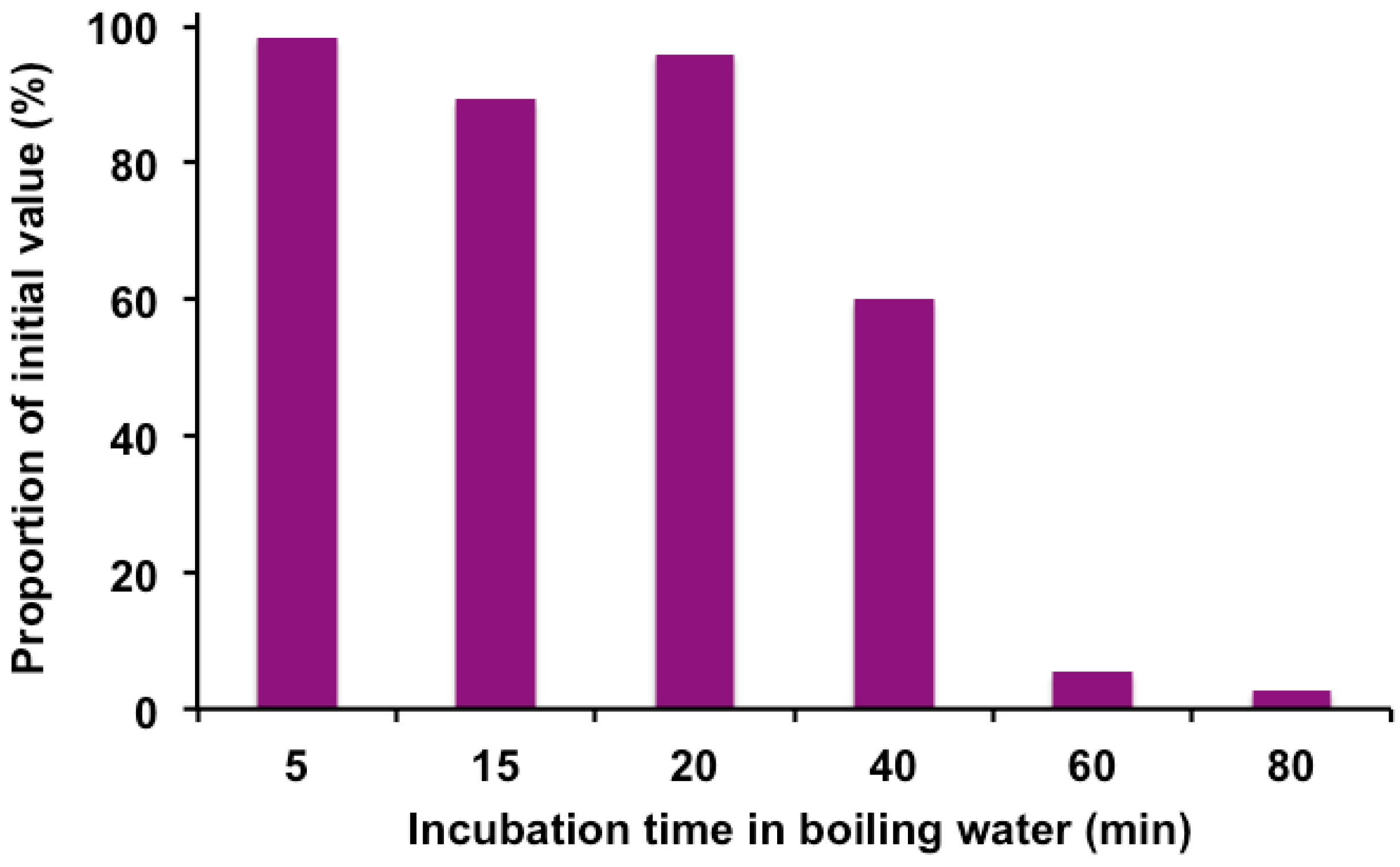
2.3. Effects of Heat Treatment on Total Phenol Content, Antioxidant and Free-Radical Scavenging Activities
2.4. Effect of Heat on Anthocyanidins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Heat Treatment of Elderberry Extracts
4.3. Pepsin Digestion
4.4. Pancreatin Digestion and Sequential Digestion
4.5. SDS-Polyacrylamide Gel Electrophoresis
4.6. Total Phenol Determination
4.7. CUPRAC Assay for Antioxidant Activity
4.8. DPPH Radical-Scavenging Activity
4.9. Total Anthocyanin Analysis
4.10. HPLC Analysis of Anthocyanins
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Hubbermann, E.V.; Heins, A.; Stöckmann, H.; Schwar, K. Influence of acids, salt, sugars and hydrocolloids on the color stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbes, T. Effects of short-term heating on total polyphenols, anthocyanins, antioxidant activity and lectins of different parts of dwarf elder (Sambucus ebulus L.). Plant Foods Hum. Nutr. 2014, 69, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, M.J.; Girbés, T. Elderberries: A source of ribosome-inactivating proteins with lectin activity. Molecules 2015, 20, 2364–2387. [Google Scholar] [CrossRef] [PubMed]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, E.; Hayashi, K.; Katayama, H.; Hayashi, T.; Obata, A. Anti-influenza virus effects of elderberry juice and its fractions. Biosci. Biotechnol. Biochem. 2012, 76, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother. Res. 2010, 24 (Suppl. 2), S129–S132. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Abdel-Wahab, Y.H.; Flatt, P.R. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J. Nutr. 2000, 130, 15–20. [Google Scholar] [PubMed]
- Bermudez-Soto, M.J.; Tomas-Barberan, F.A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur. J. Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Aricigil, S.; Pryme, I.F. Potential beneficial effects of dietary plant lectins on health. In Natural Products: Research Reviews, 1st ed.; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2014; Volume 2, pp. 1–27. [Google Scholar]
- Pusztai, A.; Bardocz, S.; Ewen, S.W.B. Uses of plant lectins in bioscience and biomedicine. Front. Biosci. 2008, 13, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.; Dorward, P.M.; Buchan, W.C.; Armour, J.C.; Pusztai, A. Consumption of diets containing raw soya beans (Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: Effects on body composition and organ weights. Br. J. Nutr. 1995, 73, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, A.; Biernat, M.; Prykhodko, O.; Kornilovska, I.; Pusztai, A.; Pierzynowski, S.G.; Björn, W.R. Induced growth and maturation of the gastrointestinal tract after Phaseolus vulgaris lectin exposure in suckling rats. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Lannoo, N.; Peumans, W.J. Advances in botanical research incorporating advances in plant pathology. Adv. Bot. Res. 2008, 48, 107–209. [Google Scholar]
- Shang, C.; Peumans, W.J.; Van Damme, E.J.M. Occurrence and taxonomical distribution of ribosome inactivating proteins belonging to the Ricin/Shiga toxin superfamily. In Ribosome-Inactivating Proteins: Ricin and Related Proteins, 1st ed.; Stirpe, F., Lappi, D., Eds.; Willy Blackwell: Oxford, UK, 2014; pp. 11–27. [Google Scholar]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Tejero, J.; Cordoba-Diaz, D.; Quinto, E.J.; Garrosa, M.; Gayoso, M.J.; Girbés, T. Ebulin from dwarf elder (Sambucus ebulus L.): A mini-review. Toxins 2015, 7, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Girbés, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Mach, L.; Scherf, W.; Ammann, M.; Poetsch, J.; Bertsch, W.; März, L.; Glössl, J. Purification and partial characterization of a novel lectin from elder (Sambucus nigra L.) fruit. Biochem. J. 1991, 278, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Kaku, H.; Peumans, W.J.; Goldstein, I.J. Isolation and characterization of a second lectin (SNA-II) present in elderberry (Sambucus nigra L.) bark. Arch. Biochem. Biophys. 1990, 277, 255–262. [Google Scholar] [CrossRef]
- Förster-Waldl, E.; Marchetti, M.; Schöll, I.; Focke, M.; Radauer, C.; Kinaciyan, T.; Nentwich, I.; Jäger, S.; Schmid, E.R.; Boltz-Nitulescu, G.; et al. Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clin. Exp. Allergy 2003, 33, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Tejero, J.; Cabrero, P.; Cordoba-Diaz, D.; Girbes, T. Differential sensitivity of d-galactose-binding lectins from fruits of dwarf elder (Sambucus ebulus L.) to a stimulated gastric fluid. Food Chem. 2013, 136, 794–902. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, D.; Girbes, T. Isolation and molecular characterization of two lectins from dwarf elder (Sambucus ebulus L.) blossoms related to the Sam n1 allergen. Toxins 2013, 5, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of food allergens to digestion in vitro. Nat. Biotech. 1996, 14, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Abbott, U.R.; Hatzos, C. Digestibility of food allergens and non-allergenic proteins in simulated gastric fluid and simulated intestinal fluid—A comparative study. J. Agric. Food Chem. 2002, 50, 7154–7160. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Cochrane, S.A.; Warner, J.O.; Hourihane, J.O. The effect of digestion and pH on the allergenicity of kiwi fruit proteins. Pediatr. Allergy Immunol. 2008, 19, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; de Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Jimenez, P.; Argüeso, P.; Farias, G.; Méndez, E.; Girbes, T. Isolation and characterization of a new non-toxic two-chain ribosome-inactivating protein from fruits of elder (Sambucus nigra L.). J. Exp. Bot. 1996, 47, 1577–1585. [Google Scholar] [CrossRef]
- Citores, L.; Iglesias, R.; Muñoz, R.; Ferreras, J.M.; Jimenez, P.; Girbes, T. Elderberry (Sambucus nigra L.) seed proteins inhibit protein synthesis and display strong immunoreactivity with rabbit polyclonal antibodies raised against the type 2 ribosome-inactivating protein nigrin b. J. Exp. Bot. 1994, 45, 513–516. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape blueberry pomace procyanidins and total anthocyanidins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Krenn, L.; Steitz, M.; Schlicht, C.; Kurth, H.; Gaedcke, F. Anthocyanin- and proanthocyanidin-rich extracts of berries in food supplements—Analysis with problems. Pharmazie 2007, 62, 803–812. [Google Scholar] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-Glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT-Food Sci. Tech. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Hong, V.; Wrolstad, R.E. Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J. Agric. Food Chem. 1990, 38, 708–715. [Google Scholar] [CrossRef]
- Pusztai, A.; Ewen, S.W.; Grant, G.; Peumans, W.J.; van Damme, E.J.; Rubio, L.; Bardocz, S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion 1990, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Jimenez, P.; Souza, A.M.; Gayoso, M.J.; Girbes, T. Occurrence of the type two ribosome-inactivating protein nigrin b in elderberry (Sambucus nigra L.) bark. Food Res. Int. 2011, 44, 2798–2805. [Google Scholar] [CrossRef]
- Sathe, S.K.; Sze-Tao, K.W.C. Effects of sodium chloride, phytate and tannin on in vitro proteolysis of phaseolin. Food Chem. 1997, 59, 253–259. [Google Scholar] [CrossRef]
- Sathe, S.K.; Sze-Tao, K.W.C.; Wolf, W.J.; Hamaker, B.R. Biochemical Characterization and in Vitro Digestibility of the Major Globulin in Cashew Nut (Anacardium occidentale). J. Agric. Food Chem. 1997, 45, 2854–2860. [Google Scholar] [CrossRef]
- Girbés, T.; Citores, L.; Ferreras, J.M.; Rojo, M.A.; Iglesias, I.; Muñoz, R.; Arias, F.J.; Calonge, M.; García, J.R.; Méndez, E. Isolation and partial characterization of nigrin b, a non-toxic novel type 2 ribosome-inactivating protein from the bark of Sambucus nigra L. Plant Mol. Biol. 1993, 22, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Terry, L.A.; Chope, G.A.; Bordonaba, J.G. Effect of water deficit irrigation and inoculation with Botrytis cinerea on strawberry (Fragaria x ananassa) fruit quality. J. Agric. Food Chem. 2007, 55, 10812–10819. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds SNA I, SNA II, SNA IV and nigrin b are available from the authors.

| EXTRACT | Time (min) | A | B | C | D |
|---|---|---|---|---|---|
| Green fruits | 0 | 4482.8 ± 94.4 | 0.79 ± 0.01 | 370.7 ± 3.1 | 83.5 ± 5.5 |
| 10 | 4314.0 ± 99.6 | 0.71 ± 0.02 | 360.8 ± 2.1 | 76.0 ± 6.8 | |
| 20 | 3993.0 ± 67.9 | 0.59 ± 0.01 | 327.0 ± 2.5 | 62.1 ± 5.6 | |
| 40 | 2110.2 ± 67.1 | 0.37 ± 0.02 | 174.4 ± 7.9 | 23.0 ± 4.6 | |
| 80 | 571.0 ± 43.5 | 0.19 ± 0.01 | 118.8 ± 18.0 | 7.5 ± 4.8 | |
| Ripe fruits | 0 | 8928.0 ± 84.4 | 9.10 ± 0.33 | 4821.4 ± 42.4 | 2704.5 ± 5.5 |
| 10 | 8795.0 ± 138.7 | 8.61 ± 0.23 | 4776.8 ± 57.5 | 2625.5 ± 6.8 | |
| 20 | 8617.0 ± 296.9 | 7.87 ± 0.30 | 4295.4 ± 247.7 | 2185.4 ± 5.6 | |
| 40 | 5491.9 ± 54.1 | 3.55 ± 0.10 | 2055.9 ± 224.1 | 746.4 ± 4.6 | |
| 80 | 2885.9 ± 111.6 | 2.92 ± 0.08 | 1380.1 ± 45.4 | 404.6 ± 4.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro. Molecules 2017, 22, 95. https://doi.org/10.3390/molecules22010095
Jiménez P, Cabrero P, Cordoba-Diaz D, Cordoba-Diaz M, Garrosa M, Girbés T. Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro. Molecules. 2017; 22(1):95. https://doi.org/10.3390/molecules22010095
Chicago/Turabian StyleJiménez, Pilar, Patricia Cabrero, Damian Cordoba-Diaz, Manuel Cordoba-Diaz, Manuel Garrosa, and Tomás Girbés. 2017. "Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro" Molecules 22, no. 1: 95. https://doi.org/10.3390/molecules22010095






