Capparis spinosa Fruit Ethanol Extracts Exert Different Effects on the Maturation of Dendritic Cells
Abstract
:1. Introduction
2. Results
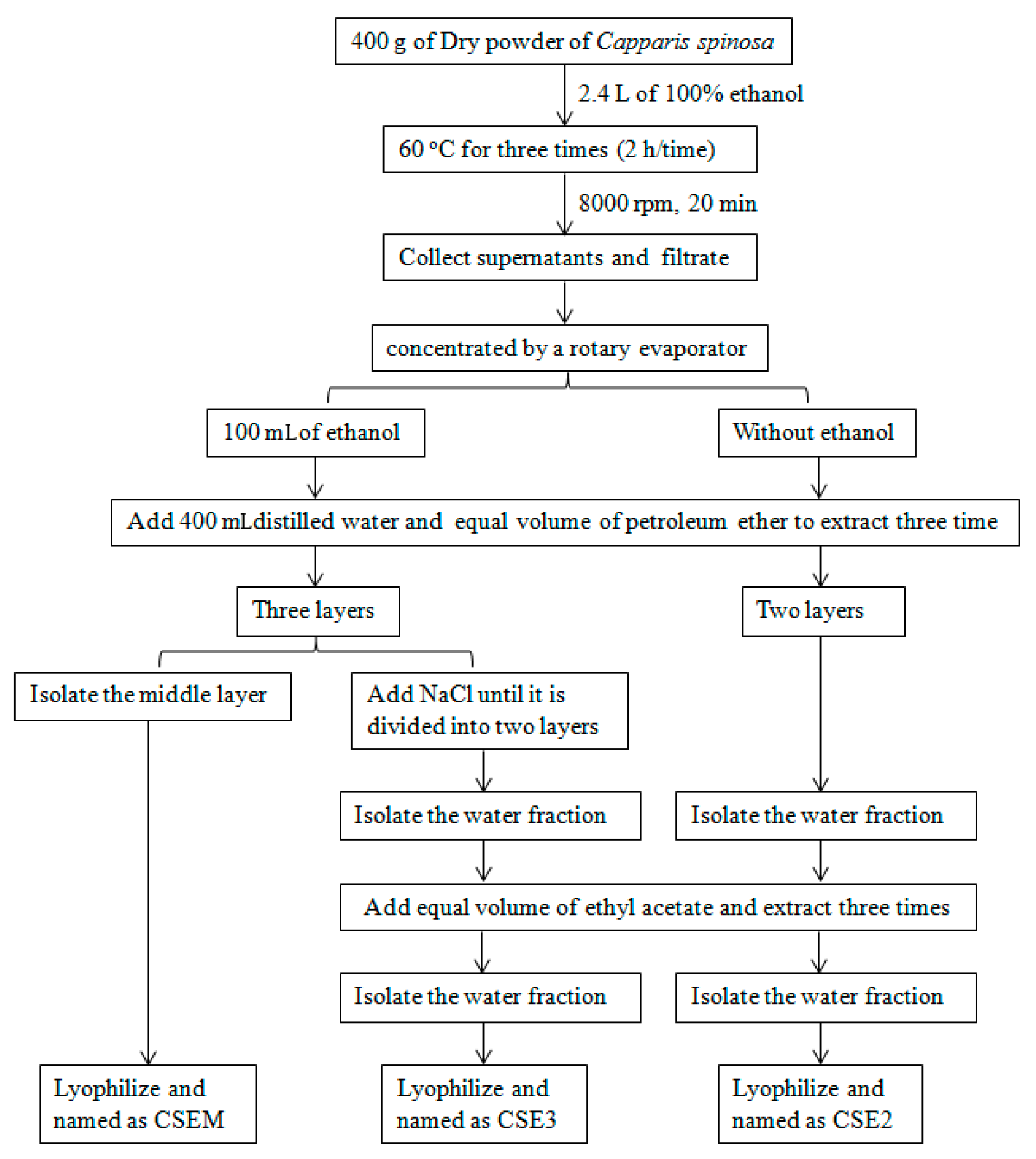
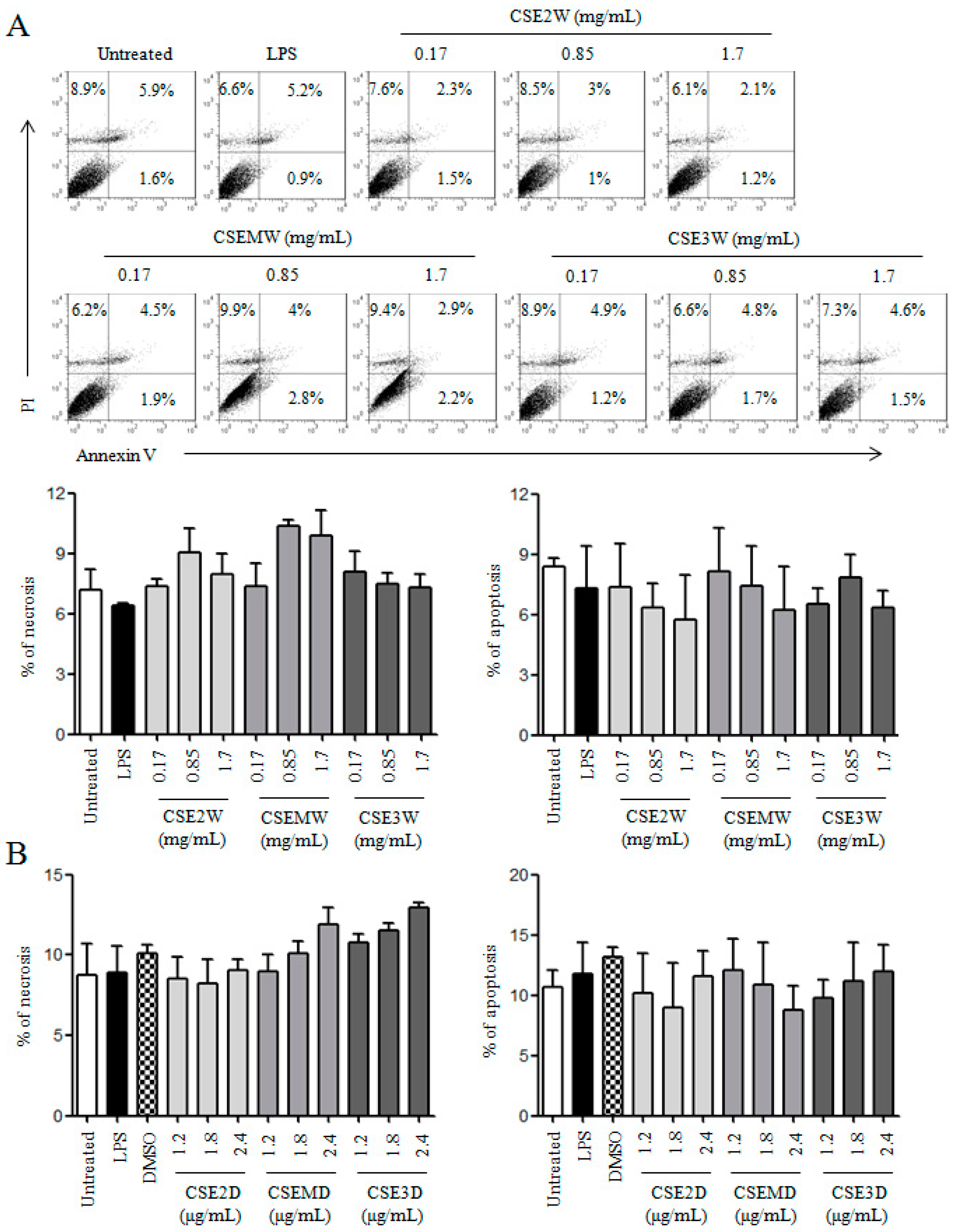
2.1. The Preparation of CSEs and Their Effect on DC Viability
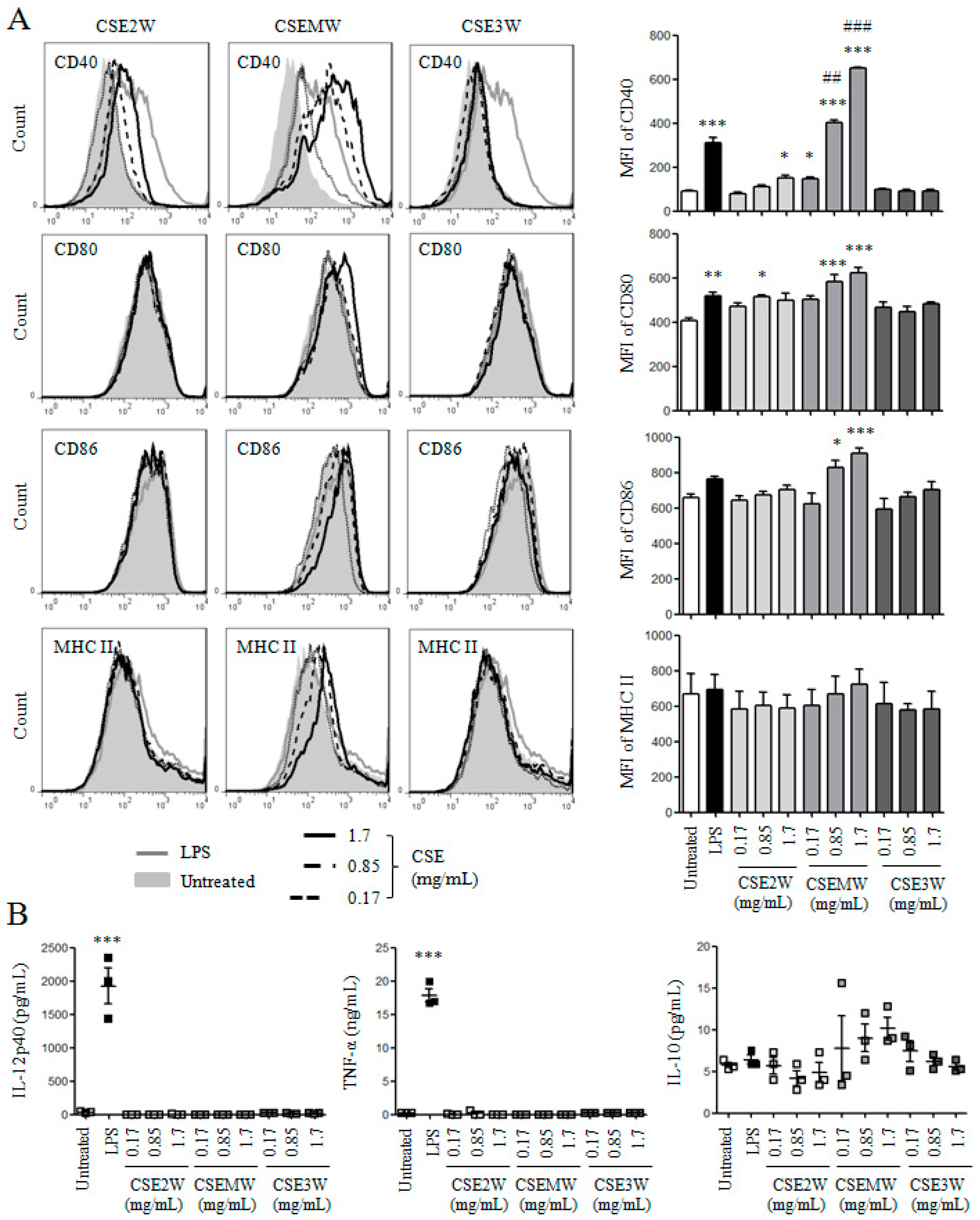
2.2. The Effect of Polysaccharides in CSEs on DC Maturation and Cytokine Production
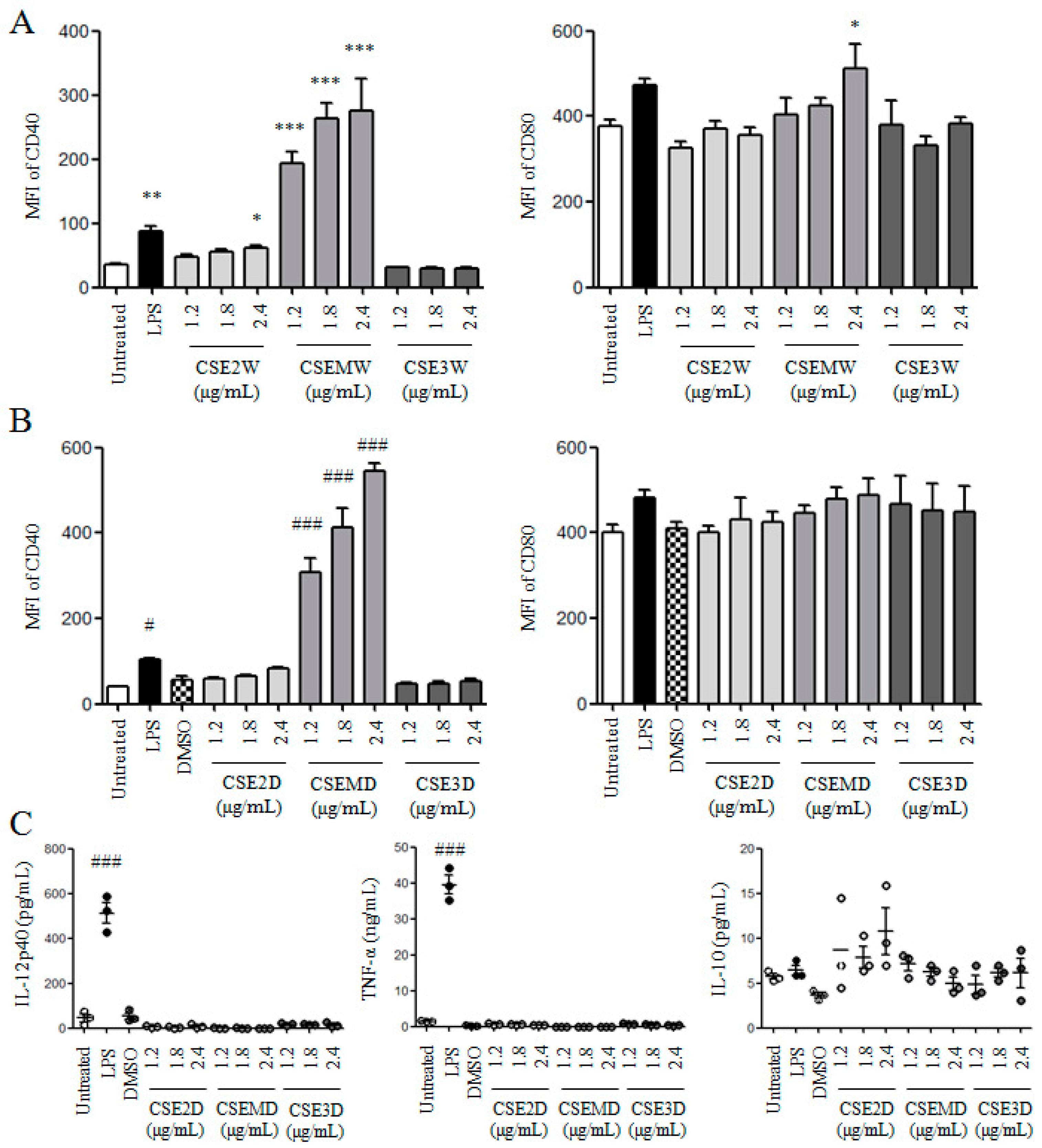
2.3. The Effect of Flavonoids in CSEs on DC Maturation and Cytokine Production
2.4. CSE3D and CSE3W Suppressed DC Maturation and Cytokine Production Induced by LPS
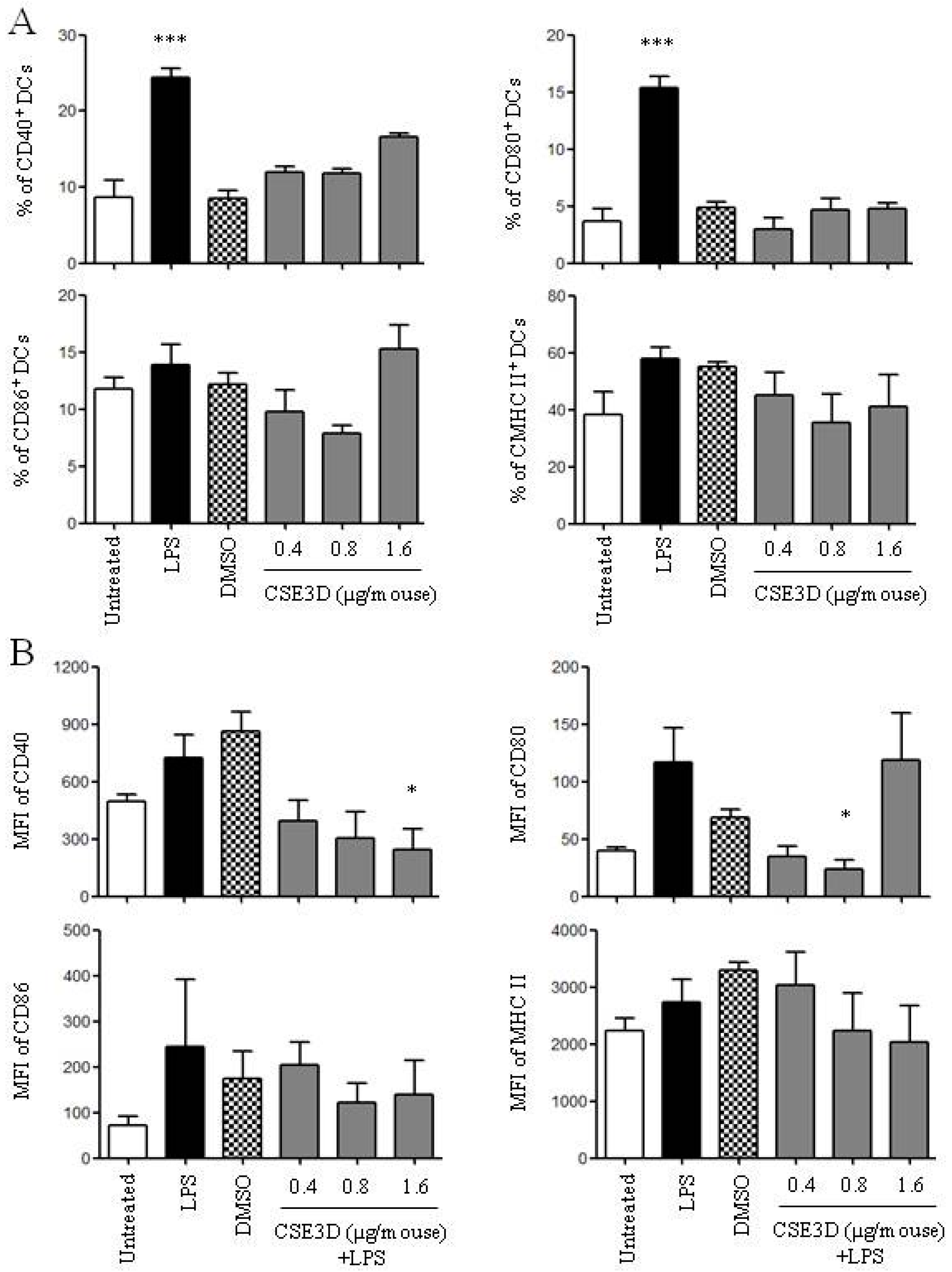
2.5. CSE3D Suppressed DC Maturation Induced by LPS In Vivo
3. Discussion
4. Materials and Methods
4.1. Preparation of C. spinosa Ethanol Extracts
4.2. Mice and Injection
4.3. The Generation of Dendritic Cell and CSE Treatment
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Flow Cytometry
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, J.; Li, J.; Zhang, F. The immunoregulatory effects of Chinese herbal medicine on the maturation and function of dendritic cells. J. Ethnopharmacol. 2015, 171, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, F.; Li, J. The immunoregulatory effects of traditional Chinese medicine on treatment of asthma or asthmatic inflammation. Am. J. Chin. Med. 2015, 43, 1059–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Howard, O.M.; Oppenheim, J.J. Dendritic cells as a pharmacological target of traditional Chinese medicine. Cell. Mol. Immunol. 2006, 3, 401–410. [Google Scholar] [PubMed]
- Huseini, H.F.; Hasani-Rnjbar, S.; Nayebi, N.; Heshmat, R.; Sigaroodi, F.K.; Ahvazi, M.; Alaei, B.A.; Kianbakht, S. Capparis spinosa L. (Caper) fruit extract in treatment of type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2013, 21, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sher, H.; Alyemeni, M.N. Ethnobotanical and pharmaceutical evaluation of Capparis spinosa L., validity of local folk and Unani system of medicine. J. Med. Plants Res. 2010, 4, 1751–1756. [Google Scholar]
- Nabavi, S.F.; Maggi, F.; Daglia, M.; Habtemariam, S.; Rastrelli, L.; Nabavi, S.M. Pharmacological Effects of Capparis spinosa L. Phytother. Res. 2016, 30, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Elfalleh, W.; Saadaoui, E.; Khaldi, A.; Triki, S.; Nasri, N. The caper (Capparis L.): ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia 2011, 82, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Gao, Y.; Yu, L. Advances in studies on constituents and their pharmacological activities of Capparis spinosa. Chin. Tradit. Herb. Drugs 2007, 38, 463–467. [Google Scholar]
- Lam, S.K.; Han, Q.F.; Ng, T.B. Isolation and characterization of a lectin with potentially exploitable activities from caper (Capparis spinosa) seeds. Biosci. Rep. 2009, 29, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. Quercetin triglyco-side from Capparis spinosa. Fitoterapia 2000, 71, 46–49. [Google Scholar] [CrossRef]
- Rodrigo, M.; Lazaro, M.J.; Alvarruiz, A.; Giner, V. Composition of capers (Capparis spinosa): Influence of cultivar, size and harvest date. J. Food Sci. 1992, 57, 1152–1154. [Google Scholar] [CrossRef]
- Khatib, M.; Pieraccini, G.; Innocenti, M.; Melani, F.; Mulinacci, N. An insight on the alkaloid content of Capparis spinosa L. root by HPLC-DAD-MS, MS/MS and (1)H qNMR. J. Pharm. Biomed. Anal. 2016, 123, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jian, R.; Kang, J.; Huang, X.; Li, Y.; Zhuang, C.; Yang, F.; Zhang, L.; Fan, X.; Wu, T.; et al. Anti-inflammatory effects of caper (Capparis spinosa L.) fruit aqueous extract and the isolation of main phytochemicals. J. Agric. Food Chem. 2010, 58, 12717–12721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Xie, C.; Jian, R.; Kang, J.; Li, Y.; Zhuang, C.L.; Yang, F.; Zhang, L.L.; Lai, L.; Wu, T.; et al. Biflavonoids from Caper (Capparis spinosa L.) fruits and their effects in inhibiting NF-kappa B activation. J. Agric. Food Chem. 2011, 59, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Moutia, M.; El Azhary, K.; Elouaddari, A.; Al Jahid, A.; Jamal Eddine, J.; Seghrouchni, F.; Habti, N.; Badou, A. Capparis spinosa L. promotes anti-inflammatory response in vitro through the control of cytokine gene expression in human peripheral blood mononuclear cells. BMC Immunol. 2016, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, N.; Shahzadeh Fazeli, S.A.; Ghafoori, H.; Farahmand, Z.; MohammadKhani, E.; Vakhshiteh, F.; Ghamarian, A.; Farhangniya, M.; Sanati, M.H. Effect of flavonoids rich extract of Capparis spinosa on inflammatory involved genes in amyloid-beta peptide injected rat model of Alzheimer’s disease. Nutr. Neurosci. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. A protein with antiproliferative, antifungal and HIV-1 reverse transcriptase inhibitory activities from caper (Capparis spinosa) seeds. Phytomedicine 2009, 16, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Bisignano, G.; Pavone, B.; Tomaino, A.; Bonina, F.P.; Saija, A.; Cristani, M.; D’Arrigo, M.; Trombetta, D. Antiviral and immunomodulatory effect of a lyophilized extract of Capparis spinosa L. buds. Phytother. Res. 2008, 22, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Occhiuto, F.; Perri, D.; Puglia, C.; Santagati, N.A.; de Pasquale, A.; Saija, A.; Bonina, F. Antiallergic and antihistaminic effect of two extracts of Capparis spinosa L. flowering buds. Phytother. Res. 2005, 19, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lu, J.; Xin, H.; Zhang, L.; Wang, Y.; Tang, K. Anti-arthritic active fraction of Capparis spinosa L. fruits and its chemical constituents. Yakugaku Zasshi 2011, 131, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.B.; Yu, L. N-butanol extract of Capparis spinosa L. induces apoptosis primarily through a mitochondrial pathway involving mPTP open, cytochrome C release and caspase activation. Asian Pac. J. Cancer Prev. 2014, 15, 9153–9157. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; Gentile, C.; Livrea, M.A. Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J. Agric. Food Chem. 2007, 55, 8465–8471. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Li, X.; Zheng, M. Capparis spinosa protects against oxidative stress in systemic sclerosis dermal fibroblasts. Arch. Dermatol. Res. 2010, 302, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Germanò, M.P.; de Pasquale, R.; D’Angelo, V.; Catania, S.; Silvari, V.; Costa, C. Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J. Agric. Food Chem. 2002, 50, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.B.; Jilani, I.B.; Bouaziz, M.; Gargouri, B.; Elloumi, N.; Attia, H.; Ghrabi-Gammar, Z.; Lassoued, S. Phenolic contents and antioxidant activity of ethanolic extract of Capparis spinosa. Cytotechnology 2016, 68, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Micheli, L.; Di Cesare Mannelli, L.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Arslan, R.; Bektas, N.; Ozturk, Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J. Ethnopharmacol. 2010, 131, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, M.; Abad, M.; Haeri, M.R.; Ebrahimi, M.; Heidari, R. Anti-diabetic effect of Capparis spinosa L. root extract in diabetic rats. Avicenna J. Phytomed. 2015, 5, 325–332. [Google Scholar] [PubMed]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Jalali, M.T.; Mohammadtaghvaei, N.; Larky, D.A. Investigating the effects of Capparis spinosa on hepatic gluconeogenesis and lipid content in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2016, 84, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Lemhadri, A.; Michel, J.B. Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J. Ethnopharmacol. 2005, 98, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bilen, S.; Altunoglu, Y.C.; Ulu, F.; Biswas, G. Innate immune and growth promoting responses to caper (Capparis spinosa) extract in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 57, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Shrivastava, P.; van Drunen Littel-van den Hurk, S. The role of dendritic cells in innate and adaptive immunity to respiratory syncytial virus, and implications for vaccine development. Expert Rev. Vaccines 2012, 11, 1441–1457. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1, 2, 3 and 4. Curr. Opin. Investig. Drugs 2009, 10, 526–535. [Google Scholar] [PubMed]
- Mei, L. The Preparation of Capparis spinosa L. Ointment on Anti-Rheumatoid Arthritis. Master’s Thesis, HuaZhong University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Agbo, M.O.; Uzor, P.F.; Akazie-Nneji, U.N.; Eze-Odurukwe, C.U.; Ogbatue, U.B.; Mbaoji, E.C. Antioxidant, total phenolic and flavonoid content of selected Nigerian medicinal plants. Dhaka Univ. J. Pharm. Sci. 2015, 14, 35–41. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Jiang, Y.; Wang, Z.J.; Sun, G.L.; Xu, D.S.; Feng, Y.; Shen, L. Poly(ethylene glycol)-Radix Ophiopogonis polysaccharide conjugates: Preparation, characterization, pharmacokinetics and in vitro bioactivity. Eur. J. Pharm. Biopharm. 2010, 76, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, B.; Li, J.; Cao, X.; Diao, M.; Feng, H.; Chen, X.; Chen, Z.; Zeng, X. Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int. Immunopharmacol. 2012, 14, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Hua, K.F.; Lin, C.C.; Lin, C.H.; Hsu, J.; Wong, C.H. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Chen, J. Polysaccharide purified from Polyporus umbellatus (Per) Fr induces the activation and maturation of murine bone-derived dendritic cells via toll-like receptor 4. Cell. Immunol. 2010, 265, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Coutant, F.; Miossec, P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat. Rev. Rheumatol. 2016, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, A.; Tadagavadi, R.K.; Swafford, D.; Manicassamy, S. Modulation of Inflammatory Responses by Wnt/β-Catenin Signaling in Dendritic Cells: A Novel Immunotherapy Target for Autoimmunity and Cancer. Front. Immunol. 2016, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Wang, W.; Luo, J.; Aipire, A.; Li, J.; Zhang, F. Pleurotus ferulae water extract enhances the maturation and function of murine bone marrow-derived dendritic cells through TLR4 signaling pathway. Vaccine 2015, 33, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

| Extracts | Polysaccharides (mg/mL) | Flavonoids (mg/mL) | Ratios of Polysaccharides/Flavonoids |
|---|---|---|---|
| CSE2W | 115.58 | 0.34 | 340 |
| CSE2D | 97.87 | 0.26 | 376 |
| CSEMW | 67.17 | 0.46 | 146 |
| CSEMD | 66.91 | 0.79 | 85 |
| CSE3W | 133.23 | 0.2 | 666 |
| CSE3D | 58.81 | 0.16 | 368 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamuti, A.; Li, J.; Zhou, F.; Aipire, A.; Ma, J.; Yang, J.; Li, J. Capparis spinosa Fruit Ethanol Extracts Exert Different Effects on the Maturation of Dendritic Cells. Molecules 2017, 22, 97. https://doi.org/10.3390/molecules22010097
Hamuti A, Li J, Zhou F, Aipire A, Ma J, Yang J, Li J. Capparis spinosa Fruit Ethanol Extracts Exert Different Effects on the Maturation of Dendritic Cells. Molecules. 2017; 22(1):97. https://doi.org/10.3390/molecules22010097
Chicago/Turabian StyleHamuti, Azeguli, Jinyu Li, Fangfang Zhou, Adila Aipire, Ji Ma, Jianhua Yang, and Jinyao Li. 2017. "Capparis spinosa Fruit Ethanol Extracts Exert Different Effects on the Maturation of Dendritic Cells" Molecules 22, no. 1: 97. https://doi.org/10.3390/molecules22010097






