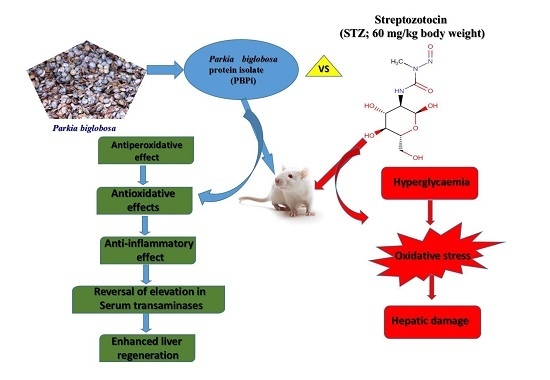
Protective Effects of Parkia biglobosa Protein Isolate on Streptozotocin-Induced Hepatic Damage and Oxidative Stress in Diabetic Male Rats
Abstract
:1. Introduction
2. Results
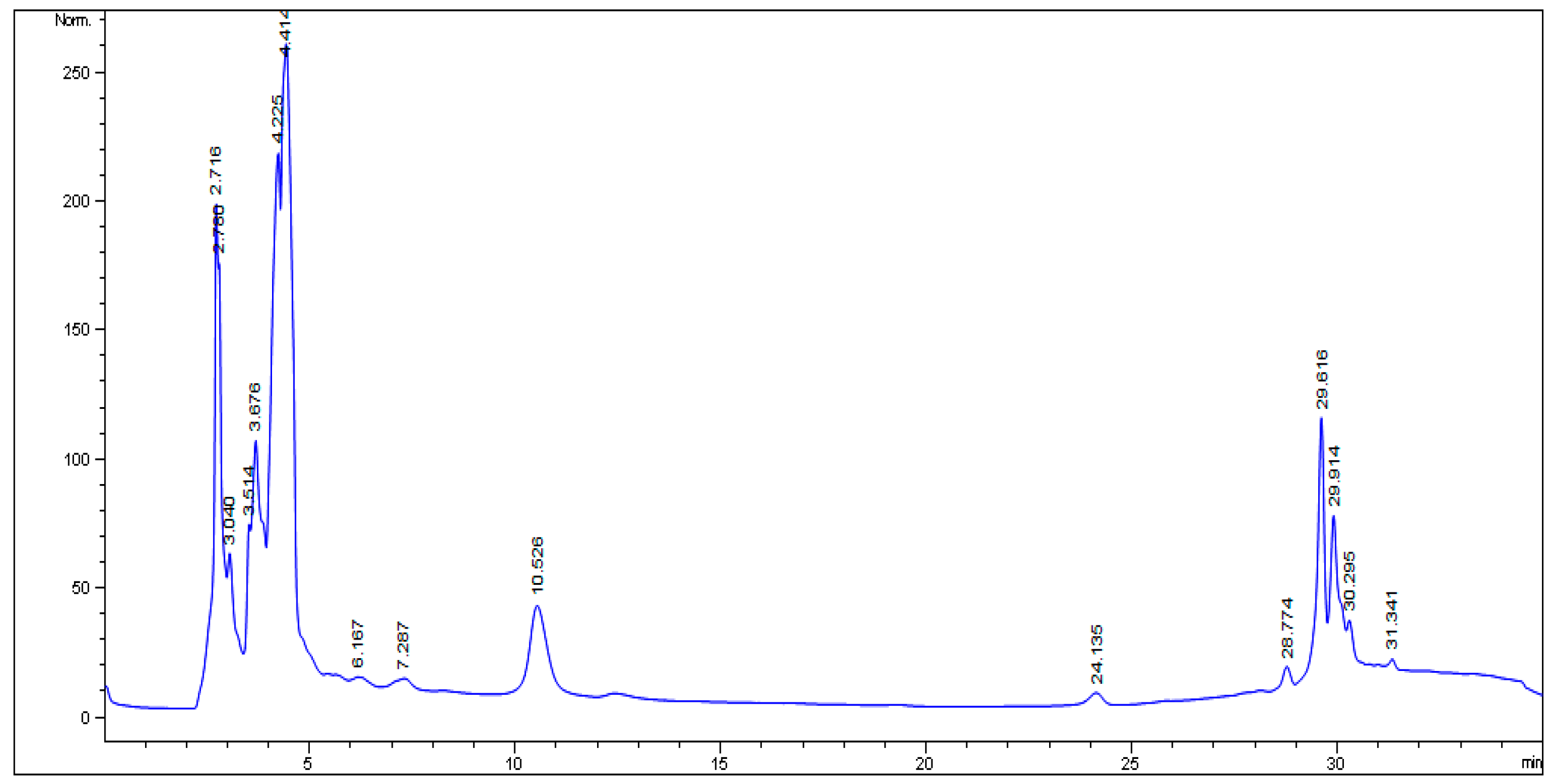
2.1. HPLC Analysis
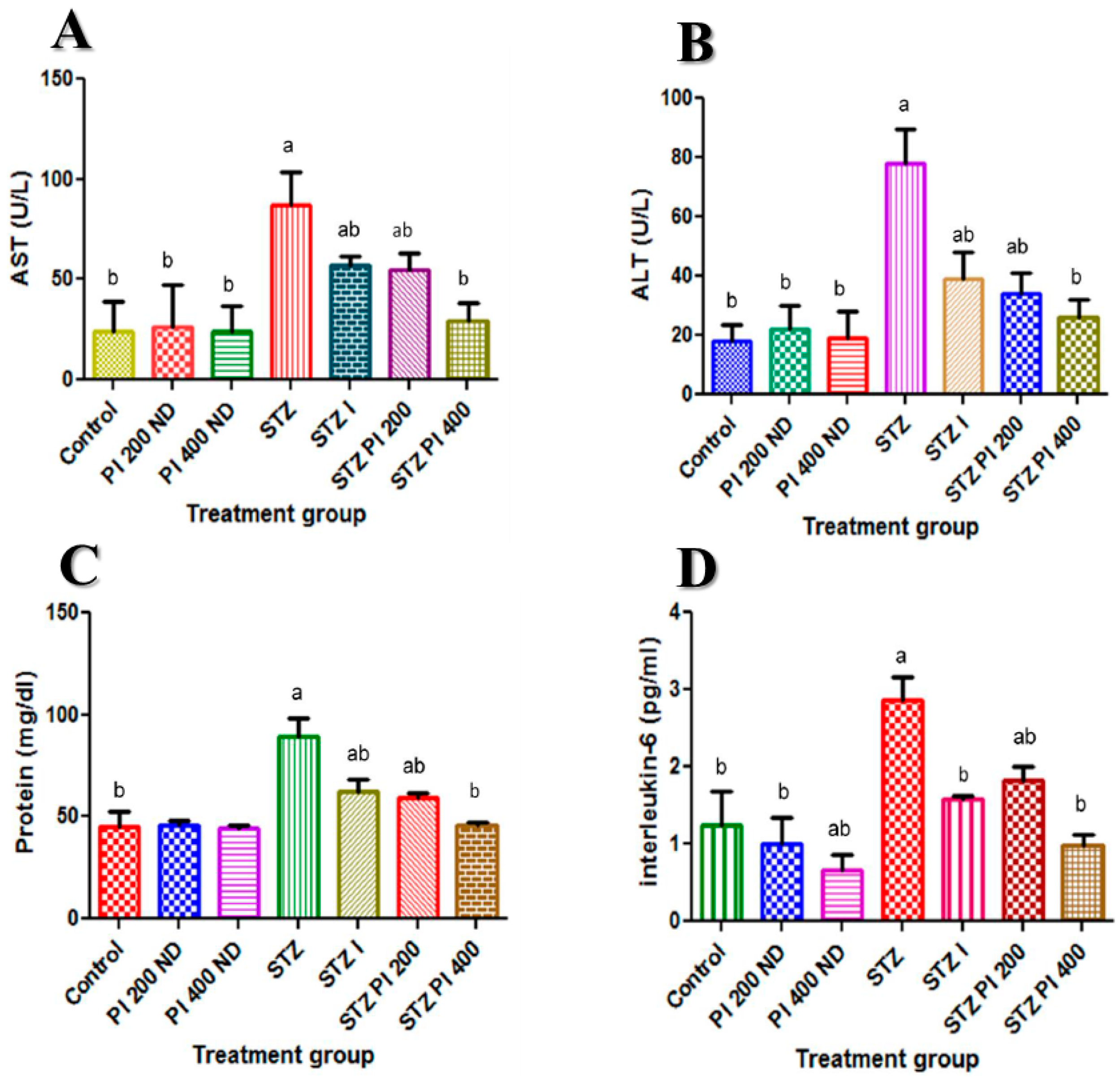
2.2. Effect of PBPi on Serum Liver Function
2.3. Effect of PBPi on Serum Interleukin-6 (IL-6)
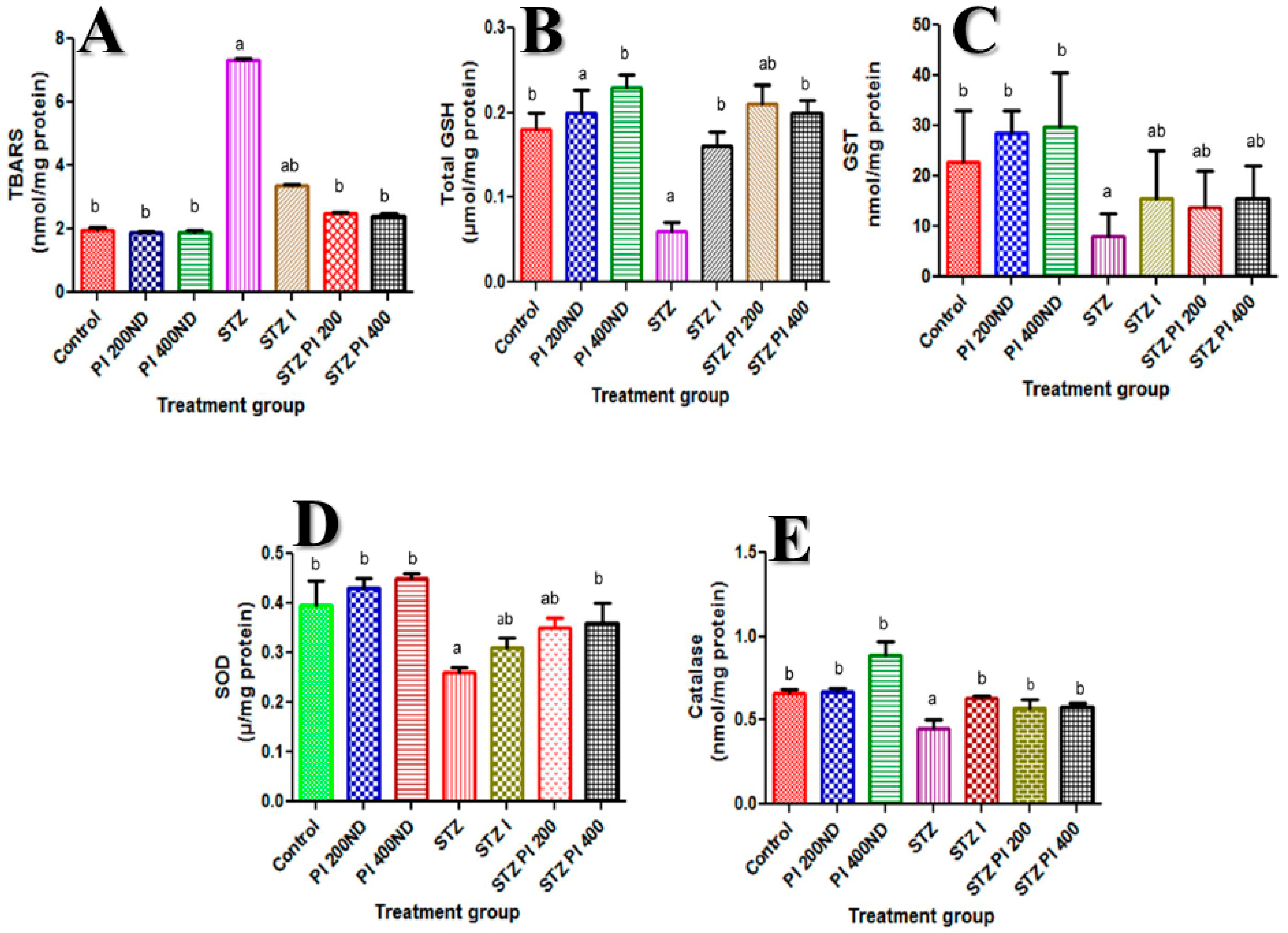
2.4. Effect of PBPi on Assessment of Oxidative Stress and Antioxidants
2.5. Effect of PBPi on Liver Histopathological Examination
3. Discussion and Conclusions
4 Materials and Methods
4.1. Chemicals
4.2. Plant Material and Preparation of the Protein Isolate
4.3. Induction of Diabetes in Experimental Animals
4.4. Experimental Design and Biochemical Analysis
4.5. HPLC Analysis
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaveeshwar, S.A.; Cornwall, J. The current state of diabetes mellitus in India. Australas. Med. J. 2014, 7, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian. Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Irudayaraj, S.S.; Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. Leaves in Streptozotocin induced diabetic rats. J. Ethnopharmacol 2012, 143, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic Foot Syndrome as a Possible Cardiovascular Marker in Diabetic Patients. J. Diabetes Res. 2015, 2015, 268390. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, M.I.; Akanji, M.A.; Yakubu, M.T.; Ashafa, A.O.T. Protective effect of free and bound polyphenol extracts from ginger (Zingiber officinale Roscoe) on the hepatic antioxidant and some carbohydrate metabolizing enzymes of streptozotocin-induced diabetic rats. Evid. Based Complement. Altern. Med. 2013, 2013, 935486. [Google Scholar] [CrossRef] [PubMed]
- Ogunyinka, B.I.; Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Potentials of some plant-derived foods in the management of diabetes and associated Complications. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 12–20. [Google Scholar] [CrossRef]
- Ahmed, D.; Kumar, V.; Verma, A.; Gupta, P.S.; Kumar, H.; Dhingra, V.; Mishra, V.; Sharma, M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement. Alternat. Med. 2014, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Karuna, R.; Bharathi, V.G.; Reddy, S.S.; Ramesh, B.; Saralakumari, D. Protective effects of Phyllanthus amarus aqueous extract against renal oxidative stress in Streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2011, 43, 414–418. [Google Scholar] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Afrin, R.; Arumugam, S.; Soetikno, V.; Thandavarayan, R.A.; Pitchaimani, V.; Karuppagounder, V.; Sreedhar, R.; Harima, M.; Suzuki, H.; Miyashita, S.; et al. Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats. Free Rad. Res. 2015, 49, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Frances, D.E.; Ingaramo, P.I.; Ronco, M.T.; Carnovale, C.E. Diabetes, an inflammatory process: Oxidative stress and TNF-alpha involved in hepatic complications. J. Biomed. Sci. Eng. 2013, 6, 645–653. [Google Scholar] [CrossRef]
- Uyanik, M.H.; Albayrak, A.; Odabasoglu, F.; Karakus, E.; Ozden, K.; Polat, B.; Yayla, M.; Karamese, M.; Albayrak, A.; Yazgi, H. Effects of diabetes on cytokines and oxidative organ injury in a rat model of sepsis. Cell. Mol. Biol. 2012, 58, 1623–1631. [Google Scholar]
- Sehgal, P.B. Interleukin-6: molecular pathophysiology. J. Invest. Dermatol. 1990, 94, 2S–6S. [Google Scholar] [CrossRef] [PubMed]
- Niture, N.T.; Ansari, A.A.; Naik, S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014, 52, 720–727. [Google Scholar] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, E.O. Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. Afr. J. Biomed. Res. 2002, 5, 125–129. [Google Scholar] [CrossRef]
- Odetola, A.A.; Akinloye, O.; Egunjobi, C.; Adekunle, W.A.; Ayoola, A.O. Possible antidiabetic and antihyperlipidaemic effect of fermented Parkia biglobosa (JACQ) extract in alloxan-induced diabetic rats. Clin. Exp. Pharmacol. Physiol. 2006, 233, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2005, 19, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Sartori, D.R.S.; Kawakami, C.L.; Orsatti, C.L.; Sforcin, J.M. Propolis effect on streptozotocin-induced diabetic rats. J. Venom. Anim. Toxins. Incl. Trop Dis. 2009, 15, 93–102. [Google Scholar] [CrossRef]
- Hassan, S.K.; El-Sammad, N.M.; Mousa, A.M.; Mohammed, M.H.; Farrag, A.E.; Hashim, A.N.; Werner, V.; Lindequist, U.; Nawwar, M.A. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac. J. Trop Biomed. 2015, 5, 462–471. [Google Scholar] [CrossRef]
- Okechukwu, P.N.; Ndyeabura, A.W.; Chiang, C.N.; Akowuah, G.A. Effect of standardized extract of Cosinium fenestratum stem bark on liver and kidney function parameters in streptozotocin-induced diabetic rats. J. Acute. Dis. 2013, 2, 201–206. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Ukwenya, V.O.; Obuotor, E.M.; Adewole, S.O. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement. Alternat. Med 2014, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.S.; Gurtu, S. Hepatoprotective effect of tualang honey supplementation in streptozotocin-induced diabetic rats. Int. J. Appl. Res. Nat. Prod. 2012, 4, 37–41. [Google Scholar]
- Luke, U.O.; Ebong, P.E.; Eyong, E.U.; Robert, A.E.; Ufot, S.U.; Egbung, G.E. Effect of ethanolic root and twig extracts of vernonia amygdalina (ETIDOT) on liver function parameters of streptozotocin induced hyperglycaemic and normal wistar rats. Eur. Sci. J. 2013, 9, 199–211. [Google Scholar]
- Pradeepa, S.; Subramanian, S.; Kaviyarasan, V. Biochemical evaluation of antidiabetic properties of Pithecellobium dulce fruits studied in streptozotocin induced experimental diabetic rats. Int. J. Herbal. Med. 2013, 1, 21–28. [Google Scholar]
- Rao, U.M.; Adinew, B. Remnant B-cell-stimulative and anti-oxidant effects of Persea americana fruit extract studied in rats introduced into streptzotocin-induced hyperglycaemic state. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.A.; Braga, C.P.; Novelli, E.L.; Fernandes, A.A. Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidant vitamin. Braz. Arch. Biol. Technol. 2012, 55, 527–536. [Google Scholar] [CrossRef]
- Balamurugan, K.; Nishanthini, A.; Mohan, V.R. Antidiabetic and antihyperlipidaemic activity of ethanol extract of Melastoma malabathricum Linn. leaf in alloxan induced diabetic rats. Asian. Pac. J. Trop Biomed. 2014, 4, S442–S448. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Dhanapal, C.K. Evaluation of in vivo in vivo antioxidant activity of methanolic extract of Coleus vettiveroides Jacob in streptozotocin-induced oxidative stress in rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 97–103. [Google Scholar]
- Al-Enazi, M.M. Combined Therapy of Rutin and Silymarin has More Protective Effects on Streptozotocin-Induced Oxidative Stress in Rats. J. Appl. Pharm. Sci. 2014, 4, 21–28. [Google Scholar]
- Al-Malki, A.L.; El Rabey, H.A. The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. BioMed. Res. Int. 2015, 2015, 381040. [Google Scholar] [CrossRef] [PubMed]
- Kuate, D.; Kengne, A.P.; Biapa, C.P.; Azantsa, B.G.; Muda, W.A. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Ebong, P.E.; Atangwho, I.J.; Eyong, E.U.; Egbung, G.E.; Ikpeme, E.V. Effect of co-administration of extracts of Vernonia amygdalina and Azadirachta indica on lipid profile and oxidative stress in hepatocytes of normal and diabetic rats. Agric. Biol. J. N. Am. 2011, 2, 1087–1095. [Google Scholar] [CrossRef]
- Shaikh, H.; Shrivastava, V.K. Effects of streptozotocin induced diabetes mellitus type 1 on the rat brain antioxidant status and activity of acetyl-cholinesterase: a novel and potential treatment by vitex negundo. Int. J. Pharm. Pharm. Sci. 2014, 6, 252–256. [Google Scholar]
- Adewole, S.; Ojewole, J. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 30–41. [Google Scholar] [CrossRef]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective Effects of d-Limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhardwaj, P.; Sharma, P. Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacognosy Res. 2013, 5, 225–232. [Google Scholar] [PubMed]
- Fatani, A.J.; Al‑Rejaie, S.S.; Abuohashish, H.M.; Al‑Assaf, A.; Parmar, M.Y.; Ola, M.S.; Ahmed, M.M. Neuroprotective effects of Gymnema sylvestre on streptozotocin-induced diabetic neuropathy in rats. Exp. Ther. Med. 2015, 9, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, V.N.; Rajasekaran, N.; Gopalakrishnan, V.K.; Devaki, K. In vivo in vivo antioxidant activity of Premna corymbosa (Rottl.) against streptozotocin induced oxidative stress in Wistar albino rats. J. App. Pharm. Sci. 2012, 2, 60–65. [Google Scholar]
- Hule, A.K.; Shah, A.S.; Gambhire, M.N.; Juvekar, A.R. An evaluation of the antidiabetic effects of Elaeocarpus ganitrus in experimental animals. Indian J. Pharmacol. 2011, 43, 56–59. [Google Scholar] [PubMed]
- Varshney, R.; Kale, R.K. Effect of Calmodulin Antagonist on Radiation-Induced Lipid Peroxidation in Microsomes. Int. J. Rad. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Loonat, F.; Amabeoku, G.J. Antinociceptive, Anti-Inflammatory and Antipyretic Activities of the Leaf Methanol Extract of Ruta Graveolens L. (Rutaceae) in Mice and Rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 173–181. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound PBPi is available from the authorsSamples of the compounds |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunyinka, B.I.; Oyinloye, B.E.; Osunsanmi, F.O.; Opoku, A.R.; Kappo, A.P. Protective Effects of Parkia biglobosa Protein Isolate on Streptozotocin-Induced Hepatic Damage and Oxidative Stress in Diabetic Male Rats. Molecules 2017, 22, 1654. https://doi.org/10.3390/molecules22101654
Ogunyinka BI, Oyinloye BE, Osunsanmi FO, Opoku AR, Kappo AP. Protective Effects of Parkia biglobosa Protein Isolate on Streptozotocin-Induced Hepatic Damage and Oxidative Stress in Diabetic Male Rats. Molecules. 2017; 22(10):1654. https://doi.org/10.3390/molecules22101654
Chicago/Turabian StyleOgunyinka, Bolajoko Idiat, Babatunji Emmanuel Oyinloye, Foluso Oluwagbemiga Osunsanmi, Andrew Rowland Opoku, and Abidemi Paul Kappo. 2017. "Protective Effects of Parkia biglobosa Protein Isolate on Streptozotocin-Induced Hepatic Damage and Oxidative Stress in Diabetic Male Rats" Molecules 22, no. 10: 1654. https://doi.org/10.3390/molecules22101654