Antibacterial and Antitubercular Activities of Cinnamylideneacetophenones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
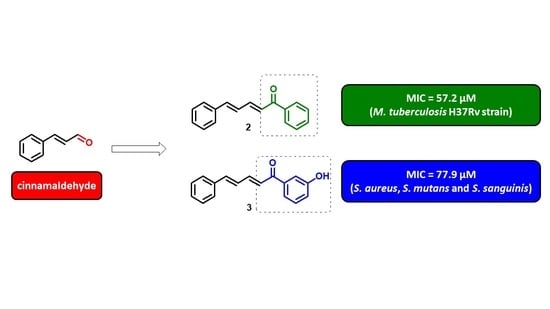
2.2. Antibacterial, Antitubercular Activity, and Structure-Activity Relationship (SAR)
2.3. Determination of Partition Coefficient Log Po/w
2.4. Evaluation of Cytotoxicity and Determination of Selectivity Index
2.5. In Silico Prediction of ADME of Cinnamylideneacetophenones
3. Materials and Methods
3.1. Synthesis of Cinnamylideneacetophenones
3.2. Antibacterial Activity of Cinnamylideneacetophenones
3.3. Antitubercular Activity of Cinnamylideneacetophenones
3.4. Partition Coefficient (log Po/w) of Cinnamylideneacetophenones
3.5. Toxic Effect on Lung Cells of Selected Cinnamylideneacetophenones
3.6. DPPH Scavenging Ability of Selected Cinnamylideneacetophenones
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al-bayati, F.A.; Mohammed, M.J. Isolation, identification, and purification of cinnamaldehyde from Cinnamomum zeylanicum bark oil. An antibacterial study. Pharm Biol. 2011, 47, 61–66. [Google Scholar] [CrossRef]
- Fabra, M.J.; Mayorga, J.L.C.; Randazzo, W.; Lagarón, J.M.; Rubio, A.L.; Aznar, R.; Sánches, G. Efficacy of cinnamaldehyde against enteric viruses and its activity after incorporation into biodegradable multilayer systems of interest in food packaging. Food Environ. Virol. 2016, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Adabiardakani, A.; Mohammad, H.H.K.; Kargar, H. Cinnamaldehyde schiff base derivatives: A short review. World Appl. Program. 2012, 2, 472–476. [Google Scholar]
- Visvalingam, J.; Palaniappan, K.; Holley, R.A. In vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control. 2017, 79, 288–291. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manu, D.; Mendonca, A.F.; Daraba, A.; Dickson, J.S.; Sebranek, J.; Shaw, A.; Wang, F.; White, S. Antimicrobial efficacy of cinnamaldehyde against Escherichia coli O157:H7 and Salmonella enterica in carrot juice and mixed berry juice held at 4 °C and 12 °C. Foodborne Pathog. Dis. 2017, 14, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, X.; Zhao, X.; Meng, R.; Liu, Z.; Chen, X.; Guo, N. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Wong, S.Y.Y.; Grant, I.R.; Friedman, M.; Elliott, C.T.; Situ, C. Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 2008, 74, 5986–5990. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.A.; Moorillón, G.V.N.; Torres, L.E.S.; García, M.V.; Ramírez, B.E.S.; Valdez, L.M.R.; Chavira, B.E.R. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Kettunem, H.; Bento, M.H.L.; Saarinen, M.; Lahtinen, S.; Ouwehand, A.C.; Schulze, H.; Rautonen, N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010, 51, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Zhou, W.; Yang, J.D.; Chen, J.; Yin, Y.F.; Shi, Z.Q. Cinnamaldehyde induces PCD-like death of Microcystis. aeruginosa via reactive oxygen species. Water Air Soil Pollut. 2011, 217, 105–113. [Google Scholar] [CrossRef]
- Adams, T.B.; Cohen, S.M.; Douli, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2004, 42, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005, 43, 799–836. [Google Scholar] [CrossRef] [PubMed]
- Venkitanarayanan, K.; Kollanoor-Johny, A.; Darre, M.J.; Donoghue, A.M.; Donoghue, D.J. Use of plant-derived antimicrobials for improving the safety of poultry products. Poult. Sci. 2013, 92, 493–501. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.; Fenner, B.P.; Buzzi, F.C.; Filho, F.V.C.; Nunes, R.J. Antinociceptive activity and preliminary structure-activity relationship of chalcone-like compounds. Z. Naturforsch. C J. Biosci. 2008, 63, 830–836. [Google Scholar] [CrossRef]
- Weldon, D.J.; Saulsbury, M.D.; Goh, J.; Rowland, L.; Campbell, P.; Robinson, L.; Miller, C.; Christian, J.; Amis, L.; Taylor, N.; et al. One-pot synthesis of cinnamylideneacetophenones and their in vitro cytotoxicity in breast cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3381–3384. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.; Kumar, A. Synthesis and anti-inflammatory activity of some novel 1,5 benzodiazepine derivatives. Asian J. Pharm. Clin. Res. 2016, 9, 63–66. [Google Scholar]
- Jin, H.; Xiang, L.; Wen, F.; Tao, K.; Liu, Q.; Hou, T. Improved synthesis of chalconoid-like compounds under ultrasound irradiation. Ultrason. Sonochem. 2008, 15, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A.; NCCLS: National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 5th ed.; CLSI Document M7-A5; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ferro, T.A.; Araujo, J.M.; Pinto, B.L.S.; Santos, J.S.; Souza, E.B.; Silva, B.L.R.; Colares, V.L.P.; Novais, T.M.G.; Filho, C.M.B.; Calixto, J.B.; et al. Cinnamaldehyde inhibits Staphylococcus aureus virulence factors and protects against infection in a Galleria mellonella model. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Maillard, J.Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 117: Partition coefficient (n-octanol/water), HPLC method. In OECD Guidelines for the Testing Chemicals; OECD Publishing: Paris, France, 2004; pp. 1–11. [Google Scholar]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G.J. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.C.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Pavan, F.R. Evaluation of cytotoxic, apoptotic, mutagenic, and chemopreventive activities of semi-synthetic esters of gallic acid. Food Chem. Toxicol. 2017, 105, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.B.; Silva, N.L.F.; Silva, G.N.S.; Silva, D.B.; Lopes, N.P.; Gnoatto, S.C.; Silva, M.V.; Macedo, A.J.; Batista, J.; Tasca, T. Caatinga plants: Natural and semi-synthetic compounds potentially active against Trichomonas vaginalis. Bioorg. Med. Chem. Lett. 2016, 26, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.S.; Zeid, A.A.; Hawary, S.S.; Sleem, A.A.; Ashour, W.E. Flavonoid constituents, cytotoxic and antioxidant activities of Gleditsia. triacanthos L. leaves. Saudi J. Biol. Sci. 2014, 21, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, P.; Yu, H.; Na, Z.; Wang, J. Scavenging ability of dendritic PAMAM bridged hindered phenolic antioxidants towards DPPH˙ and ROO˙ free radicals. RSC Adv. 2017, 7, 1869–1876. [Google Scholar] [CrossRef]
- Regasini, L.O.; Vellosa, J.C.R.; Silva, D.H.S.; Furlan, M.; Oliveira, O.M.M.; Khalil, N.M.; Brunetti, I.L.; Young, M.C.M.; Barreiro, E.J.; Bolzani, V.S. Flavonols from Pterogyne. nitens and their evaluation as myeloperoxidase inhibitors. Phytochemistry 2008, 69, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Nazaré, A.C.; Faria, C.M.; Chiari, B.G.; Petrônio, M.S.; Regasini, L.O.; Silva, D.H.S.; Corrêa, M.A.; Isaac, V.L.; Fonseca, L.M.; Ximenes, V.F. Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams. Molecules 2014, 19, 8124–8139. [Google Scholar] [CrossRef] [PubMed]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Janneta, H.B. Antioxidant and anti-acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polkam, N.; Ramaswamy, V.R.; Rayam, P.; Allaka, T.R.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Gandamalla, D.; Yellu, N.R.; Balasubramanian, S.; et al. Synthesis, molecular properties prediction and anticancer, antioxidant evaluation of new edaravone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: www.molinspiration.com (accessed on 20 July 2017).
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Olin, M.; Baker, C.A.; Molitor, T.W.; Peterson, P.K. Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin. Microbiol. Rev. 2008, 21, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.S.; Cox, J.S.; Jacobs, W.R. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 2000, 5, 717–727. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |



| Entry | Name | R | Yield (%) |
|---|---|---|---|
| 2 | (2E,4E)-1,5-diphenylpenta-2,4-dien-1-one | H | 33 |
| 3 | (2E,4E)-1-(3-hydroxyphenyl)-5-phenylpenta-2,4-dien-1-one | m-OH | 91 |
| 4 | (2E,4E)-1-(4-hydroxyphenyl)-5-phenylpenta-2,4-dien-1-one | p-OH | 82 |
| 5 | (2E,4E)-1-(3-aminophenyl)-5-phenylpenta-2,4-dien-1-one | m-NH2 | 35 |
| 6 | (2E,4E)-1-(3-methoxyphenyl)-5-phenylpenta-2,4-dien-1-one | m-OMe | 78 |
| 7 | (2E,4E)-1-(4-methoxyphenyl)-5-phenylpenta-2,4-dien-1-one | p-OMe | 35 |
| 8 | (2E,4E)-1-(3,4-methylenedioxy)-5-phenylpenta-2,4-dien-1-one | -OCH2O- | 83 |
| 9 | (2E,4E)-1-(4-hydroxy-3-methoxyphenyl)-5-phenylpenta-2,4-dien-1-one | m-OMe,p-OH | 98 |
| 10 | (2E,4E)-1-(4-fluorophenyl)-5-phenylpenta-2,4-dien-1-one | p-F | 71 |
| 11 | (2E,4E)-1-(4-chlorophenyl)-5-phenylpenta-2,4-dien-1-one | p-Cl | 72 |
| 12 | (2E,4E)-1-(4-bromophenyl)-5-phenylpenta-2,4-dien-1-one | p-Br | 86 |
| 13 | (2E,4E)-1-(3,4-dichlorophenyl)-5-phenylpenta-2,4-dien-1-one | m,p-diCl | 79 |
| 14 | (2E,4E)-1-(3-(trifluoromethyl)phenyl)-5-phenylpenta-2,4-dien-1-one | m-CF3 | 88 |
| 15 | (2E,4E)-1-(3-nitrophenyl)-5-phenylpenta-2,4-dien-1-one | m-NO2 | 85 |
| 16 | (2E,4E)-1-(4-nitrophenyl)-5-phenylpenta-2,4-dien-1-one | p-NO2 | 84 |
| 17 | (2E,4E)-1-(3-methylphenyl)-5-phenylpenta-2,4-dien-1-one | m-Me | 49 |
| 18 | (2E,4E)-1-(4-methylphenyl)-5-phenylpenta-2,4-dien-1-one | p-Me | 53 |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | R | Sa * | Sm * | Ss * | Pa * | Ec * | Mt * | log Po/w | |||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MIC | MIC | |||
| 1 | - | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | - |
| 2 | H | >500 | - | >500 | - | >500 | - | >500 | >500 | 57.2 | 3.2 |
| 3 | m-OH | 77.9 | 156 | 77.9 | 156 | 77.9 | 156 | >500 | >500 | 88.8 | 2.7 |
| 4 | p-OH | 77.9 | 77.9 | 156 | 312 | 156 | 312 | >500 | >500 | 81.9 | 2.6 |
| 5 | m-NH2 | >500 | - | 313 | 313 | >500 | - | >500 | >500 | >200 | 2.5 |
| 6 | m-OMe | 296 | 296 | >500 | - | >500 | - | >500 | >500 | >200 | 3.3 |
| 7 | p-OMe | >500 | - | >500 | - | >500 | - | >500 | >500 | 66.6 | 3.2 |
| 8 | -OCH2O- | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.2 |
| 9 | m-OMe,p-OH | 279 | 279 | >500 | - | >500 | - | >500 | >500 | 83.5 | 2.7 |
| 10 | p-F | >500 | - | >500 | - | >500 | - | >500 | >500 | 67.4 | 3.3 |
| 11 | p-Cl | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.7 |
| 12 | p-Br | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.8 |
| 13 | m,p-diCl | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 4.1 |
| 14 | m-CF3 | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.7 |
| 15 | m-NO2 | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.3 |
| 16 | p-NO2 | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.2 |
| 17 | m-Me | >500 | - | >500 | - | >500 | - | >500 | >500 | >200 | 3.6 |
| 18 | p-Me | >500 | - | >500 | - | >500 | - | >500 | >500 | 70.9 | 3.5 |
| PEN | - | - | - | - | - | 0.4 | 0.4 | - | 118 | - | - |
| VAN | - | 3.3 | 6.8 | - | - | 3.3 | 3.3 | - | 53.8 | - | - |
| CHX | - | - | - | 2.4 | 4.7 | 4.7 | 9.5 | 19.4 | - | - | - |
| ISO | - | - | - | - | - | - | - | - | - | 0.3 | - |
| Entry | MRC-5 | SI | A549 | SI | ||
|---|---|---|---|---|---|---|
| IC50 * | Sa | Mt | IC50 * | Sa | Mt | |
| 3 | 46.3 | 0.6 | 0.5 | 96.7 | 1.2 | 1.2 |
| 4 | 53.1 | 0.7 | 0.6 | 77.5 | 1.0 | 0.9 |
| doxorubicin | 0.7 | - | - | 1.8 | - | - |
| Entry | %ABS | TPSA (Å2) | milog P | MW | HBD | HBA | Lipinski’s Violations | NROBT | Veber’s Violations |
|---|---|---|---|---|---|---|---|---|---|
| Ro5 | - | - | ≤5.00 | <500 | ≤5 | ≤10 | 1 | - | - |
| Vb | - | ≤140 | - | - | - | - | - | ≤10 | 0 |
| 1 | 100 | 17.07 | 2.48 | 132.16 | 1 | 0 | 0 | 2 | 0 |
| 2 | 100 | 17.07 | 4.33 | 234.30 | 1 | 0 | 0 | 4 | 0 |
| 3 | 96.1 | 37.30 | 3.83 | 250.30 | 2 | 1 | 0 | 4 | 0 |
| 4 | 96.1 | 37.30 | 3.85 | 250.30 | 2 | 1 | 0 | 4 | 0 |
| 5 | 94.1 | 43.09 | 3.38 | 249.31 | 2 | 2 | 0 | 4 | 0 |
| 6 | 99.9 | 26.30 | 4.36 | 264.32 | 2 | 0 | 0 | 5 | 0 |
| 7 | 99.9 | 26.30 | 4.39 | 263.32 | 2 | 0 | 0 | 5 | 0 |
| 9 | 92.9 | 46.53 | 3.67 | 280.32 | 3 | 1 | 0 | 5 | 0 |
| 10 | 100 | 17.07 | 4.49 | 252.29 | 1 | 0 | 0 | 4 | 0 |
| 18 | 100 | 17.07 | 4.78 | 248.32 | 1 | 0 | 0 | 4 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polaquini, C.R.; Torrezan, G.S.; Santos, V.R.; Nazaré, A.C.; Campos, D.L.; Almeida, L.A.; Silva, I.C.; Ferreira, H.; Pavan, F.R.; Duque, C.; et al. Antibacterial and Antitubercular Activities of Cinnamylideneacetophenones. Molecules 2017, 22, 1685. https://doi.org/10.3390/molecules22101685
Polaquini CR, Torrezan GS, Santos VR, Nazaré AC, Campos DL, Almeida LA, Silva IC, Ferreira H, Pavan FR, Duque C, et al. Antibacterial and Antitubercular Activities of Cinnamylideneacetophenones. Molecules. 2017; 22(10):1685. https://doi.org/10.3390/molecules22101685
Chicago/Turabian StylePolaquini, Carlos R., Guilherme S. Torrezan, Vanessa R. Santos, Ana C. Nazaré, Débora L. Campos, Laíza A. Almeida, Isabel C. Silva, Henrique Ferreira, Fernando R. Pavan, Cristiane Duque, and et al. 2017. "Antibacterial and Antitubercular Activities of Cinnamylideneacetophenones" Molecules 22, no. 10: 1685. https://doi.org/10.3390/molecules22101685