Effects of Sorghum Malting on Colour, Major Classes of Phenolics and Individual Anthocyanins
Abstract
:1. Introduction
- To determine the effects of malting on the abundance of total phenolics, total flavonoid content, flavan-4-ols, total anthocyanins, as well as on colour;
- To characterize and quantify the effects of malting on 3-deoxyanthocyanins.
2. Results and Discussion
2.1. Evaluation of Colour
2.2. Evaluation of Tannin Content
2.3. Evaluation of Total Phenolic Content (TPC)
2.4. Evaluation of Flavan-4-ols
2.5. Evaluation of Anthocyanins and Total Flavonoids
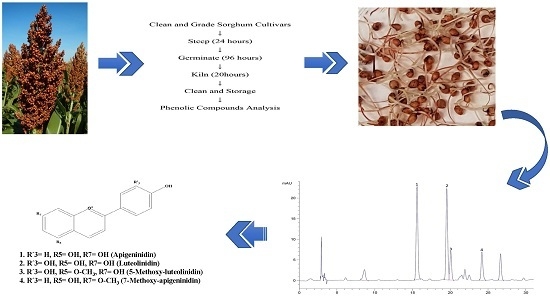
3-Deoxyanthocyanins in Raw and Malted Sorghum Grains
3. Materials and Methods
3.1. Grain Malting
3.2. Chemical Reagents
3.3. Colour Measurement
3.4. Total Phenolic Content
3.5. Condensed Tannin
3.6. Flavan-4-ols, Total Anthocyanins and Total Flavonoids
3.7. Quantification and Identification of 3-Deoxyanthocyanins Using HPLC
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Department of Agriculture. Grain: World Markets and Trade. Available online: https://www.fas.usda.gov/data/grain-world-markets-and-trade (accessed on 15 February 2016).
- ICRISAT International Crops Reseach Institute for The Semi-Arid Tropics. Available online: http://www.icrisat.org//?s=sorghum+2009 (accessed on 30 December 2009).
- Australian Bureau of Statistic Agricultural Commodities, Australia. Available online: http://www.abs.gov.au/ausstats/[email protected]/Latestproducts/7121.0Main Features412015-16# (accessed on 15 June 2016).
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; Volume 5, pp. 359–384. [Google Scholar]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.-L. Principal phenolic phytochemicals in french syrah and grenache rhone wines and their antioxidant activity in inhibiting oxidation of human low density lipoproteins. J. Int. Sci. Vigne du Vin 1995, 29, 205–212. [Google Scholar]
- Ito, N.; Fukushima, S.; Hasegawa, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J. Natl. Cancer Inst. 1983, 70, 343–347. [Google Scholar] [PubMed]
- DellaGreca, M.; Cutillo, F.D.; Abrosca, B.; Fiorentino, A.; Pacifico, S.; Zarrelli, A. Antioxidant and radical scavenging properties of Malva sylvestris. Nat. Prod. Commun. 2009, 4, 893–896. [Google Scholar] [PubMed]
- Awika, J.M. Antioxidant Properties of Sorghum. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2003. [Google Scholar]
- Rhodes, M.; Price, K. Identification and analysis of plant phenolic antioxidants. Eur. J. Cancer Prev. 1997, 6, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.; Garner, P.L.; Mayer, E.P.; Hargrove, J.L.; Hartle, D.K.; Greenspan, P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J. Med. Food 2010, 13, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of hyaluronidase activity by select sorghum brans. J. Med. Food 2008, 11, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.; Hartle, D.; Hargrove, J.; Greenspan, P. A novel nutraceutical property of select sorghum (Sorghum bicolor) brans: Inhibition of protein glycation. Phyther. Res. 2008, 22, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K.; Dowd, C. Inhibition of aromatase and α-amylase by flavonoids and proanthocyanidins from Sorghum bicolor bran extracts. J. Med. Food 2011, 14, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.R.; Reddivari, L.; Vanamala, J. The dermal layer of sweet sorghum (Sorghum bicolor) stalk, a byproduct of biofuel production and source of unique 3-Deoxyanthocyanidins, has more antiproliferative and proapoptotic activity than the pith in p53 variants of HCT116 and colon cancer stem cel. J. Agric. Food Chem. 2014, 62, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Wu, H.-T.; Tan, G.-G.; Zhu, Z.-Y.; Chai, Y.-F. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Anal. 2011, 1, 235–245. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Qin, P.; Yao, Y.; Meng, X.; Zou, J.; Zhu, K.; Ren, G. Chemical characterization of a procyanidinrich extract from sorghum bran and its effect on oxidative stress and tumor inhibition in vivo. J. Agric. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Kim, M.-J.; Park, D.-S.; Moon, H.-I. Inhibition effects of the classical pathway complement of three Sorghum bicolor from South Korea. Immunopharm. Immunot. 2011, 33, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Oladiji, A.T.; Jacob, T.O.; Yakubu, M.T. Anti-anaemic potentials of aqueous extract of Sorghum bicolor (L.) moench stem bark in rats. J. Ethnopharmacol. 2007, 111, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Akomolafe, T.L.; Adetuyi, A.O. Inhibition of cyclophosphamide-induced oxidative stress in brain by dietary inclusion of red dye extracts from sorghum (Sorghum bicolor) stem. J. Med. Food 2010, 13, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Nwinyi, F.C.; Kwanashie, H.O. Evaluation of Sorghum bicolor leaf base extract for gastrointestinal effects. Afr. J. Biotechnol. 2009, 8, 5985–5994. [Google Scholar]
- Mohamed, S.K.; Ahmed, A.A.A.; Yagi, S.M.; El, A.; Abd, W.H. Antioxidant and antibacterial activities of total polyphenols isolated from Pigmented Sorghum (Sorghum bicolor) Lines. J. Genet. Eng. Biotechnol. 2009, 7, 51–58. [Google Scholar]
- Iqbal, Z.; Munir, M.; Khan, M.; Akhtar, M.; Javed, I.; Of, O. In Vitro inhibitory effects of Sorghum bicolor on hatching and moulting of haemonchus contortus eggs. Prospects 2001, 3, 451–453. [Google Scholar]
- Taylor, J.; Dewar, J. Developments in sorghum food technologies. Adv. Food Nutr. Res. 2001, 43, 217–264. [Google Scholar] [PubMed]
- Glennie, C.W. Polyphenol changes in sorghum grain during malting. J. Agric. Food Chem. 1983, 31, 1295–1299. [Google Scholar] [CrossRef]
- Obizoba, I.C.; Atii, J.V. Effect of soaking, sprouting, fermentation and cooking on nutrient composition and some anti-nutritional factors of sorghum (Guinesia) seeds. Plant Foods Hum. Nutr. 1991, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Elmaki, H.B.; Babiker, E.E.; El Tinay, A.H. Changes in chemical composition, grain malting, starch and tannin contents and protein digestibility during germination of sorghum cultivars. Food Chem. 1999, 64, 331–336. [Google Scholar] [CrossRef]
- Towo, E.E.; Svanberg, U.; Ndossi, G.D. Effect of grain pre-treatment on different extractable phenolic groups in cereals and legumes commonly consumed in Tanzania. J. Sci. Food Agric. 2003, 83, 980–986. [Google Scholar] [CrossRef]
- Chavan, J.K.; Kadam, S.S.; Salunkhe, D.K. Changes in tannin, free amino acids, reducing sugars, and starch during seed germination of low and high tannin cultivars of sorghum. J. Food Sci. 1981, 46, 638–639. [Google Scholar] [CrossRef]
- Osuntogun, B.A.; Adewusi, S.R.A.; Ogundiwin, J.O.; Nwassike, C.C.; Nwasike, C.C. Effect of cultivar, steeping, and malting on tannin, total polyphenol, and cyanide content of nigerian sorghum. Development 1989, 66, 87–89. [Google Scholar]
- Bvochora, J.M.; Reed, J.D.; Read, J.S.; Zvauya, R. Effect of fermentation processes on proanthocyanidins in sorghum during preparation of Mahewu, a non-alcoholic beverage. Process Biochem. 1999, 35, 21–25. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Mahgoub, S.a.; Babiker, B.E. Changes in tannin and cyanide contents and diastic activity during germination and the effect of traditional processing on cyanide content of sorghum cultivars. Food Chem. 1996, 56, 159–162. [Google Scholar] [CrossRef]
- Nwanguma, B.C.; Eze, M. Changes in the concentrations of the polyphenolic constituents of sorghum during malting and mashing. J. Sci. Food Agric. 1996, 70, 162–166. [Google Scholar] [CrossRef]
- Kayodé, A.P.P.; Hounhouigan, J.D.; Nout, M.J.R. Impact of brewing process operations on phytate, phenolic compounds and in vitro solubility of iron and zinc in opaque sorghum beer. LWT-Food Sci. Technol. 2007, 40, 834–841. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Atkinson, J.I.; Leeson, S. Sorghum tannins: A review. World Poulty Sci. J. 1997, 53, 5–21. [Google Scholar] [CrossRef]
- Emmambux, N.M.; Taylor, J.R.N. Sorghum kafirin interaction with various phenolic compounds. J. Sci. Food Agric. 2003, 83, 402–407. [Google Scholar] [CrossRef]
- Wong, J.H.; Marx, D.B.; Wilson, J.D.; Buchanan, B.B.; Lemaux, P.G.; Pedersen, J.F. Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci. 2010, 179, 598–611. [Google Scholar] [CrossRef]
- Waniska, R.D.; Hugo, L.F.; Rooney, L.W. Methods To Determine the Presence of Tannins. J. Appl. Poult. Res. 1992, 1, 122–128. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic compounds and antioxidant activity of sorghum grain of varying genotype. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef] [PubMed]
- Awika, J. Sorghum Phenols as an Antioxidants. Master Thesis, Texas A & M University, College Station, TX, USA, 2000. [Google Scholar]
- Boren, B.; Waniska, R.D. Sorghum seed color as an indicator of tannin content. J. Appl. Poult. Reseach 1992, 1, 117–121. [Google Scholar] [CrossRef]
- Yang, L. Chemopreventive Potential of Sorghum with Different Phenolic Profile. Master Thesis, Texas A & M University, College Station, TX, USA, December 2009. [Google Scholar]
- Dykes, L. Flavonoid Composition and Antioxidant Activity of Pigmented Sorghum of of Varying Genotypes. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, May 2008. [Google Scholar]
- Njongmeta, N. Extractability Profiling and Antioxidant Activity of Flavonoids in Sorghum Grain and Non Grain Materials. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, May 2009. [Google Scholar]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Iwuoha, C.I.; Aina, J.O. Effects of steeping condition and germination time on the alpha-amylase activity, phenolics content and malting loss of Nigerian local red and hybrid short Kaura sorghum malts. Food Chem. 1997, 58, 289–295. [Google Scholar] [CrossRef]
- Phattanakulkaewmorie, N.; Paseephol, T.; Moongngarm, A. Chemical Compositions and Physico-Chemical Properties of Malted Sorghum Flour and Characteristics of Gluten Free Bread. Enzyme 2011, 5, 454–460. [Google Scholar]
- Ramadan, B.R.; Sorour, M.A.; Kelany, M.A. Changes in total phenolics and DPPH scavenging activity during domestic processing in some cereal grains. Ann. Food Sci. Technol. 2012, 13, 190–196. [Google Scholar]
- Dicko, M.H.; Gruppen, H.; Traore, A.S.; Van Berkel, W.J.H.; Voragen, A.G.J. Evaluation of the effect of germination on phenolic compounds and antioxidant activities in sorghum varieties. J. Agric. Food Chem. 2005, 53, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Towo, E.; Svanberg, U. Oxidation of polyphenols in phytate-reduced high-tannin cereals: Effect on different phenolic groups and on in vitro accessible iron. J. Agric. Food Chem. 2001, 49, 5630–5638. [Google Scholar] [CrossRef] [PubMed]
- Melake-Berhan, A.; Butler, L.G.; Ejeta, G.; Menkir, A. Grain mold resistance and polyphenol accumulation in sorghum. J. Agric. Food Chem. 1996, 44, 2428–2434. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Barro, C.; Traore, A.S.; Van Berkel, W.J.H.; Voragen, A.G.J. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671–2688. [Google Scholar] [CrossRef] [PubMed]
- Wharton, P.S.; Nicholson, R.L. Temporal synthesis and radiolabelling of the sorghum 3-deoxyanthocyanidin phytoalexins and the anthocyanin, cyanidin 3-dimalonyl glucoside. New Phytol. 2000, 145, 457–469. [Google Scholar] [CrossRef]
- Menkir, A.; Ejeta, G.; Butler, L.; Melakeberhan, A. Physical and chemical kernel properties associated with resistance to grain mold in sorghum. Cereal Chem. 1996, 73, 613–617. [Google Scholar]
- Audilakshmi, S.; Stenhouse, J.; Reddy, T.; Prasad, M. Grain mould resistance and associated characters of sorghum genotypes. Euphytica 1999, 107, 91–103. [Google Scholar] [CrossRef]
- Haslam, E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Haslam, E., Ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Shipp, J.; Abdel-Aal, E.-S.M. Food Applications and Physiological Effects of Anthocyanins as Functional Food Ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Kim, M.J.; Hyun, J.N.; Kim, J.A.; Park, J.C.; Kim, M.Y.; Kim, J.G.; Lee, S.J.; Chun, S.C.; Chung, I.M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Bridgers, E.N.; Chinn, M.S.; Truong, V.-D. Extraction of anthocyanins from industrial purple-fleshed sweetpotatoes and enzymatic hydrolysis of residues for fermentable sugars. Ind. Crop Prod. 2010, 32, 613–620. [Google Scholar] [CrossRef]
- Wegener, C.B.; Jansen, G.; Jurgens, H.U.; Schutze, W. Special quality traits of coloured potato breeding clones: Anthocyanins, soluble phenols and antioxidant capacity. J. Sci. Food Agric. 2009, 89, 206–215. [Google Scholar] [CrossRef]
- Taylor, J.R.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.-M.; El-beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A.; El-salam, S.M.A.; Omran, A.A. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac. J. Trop. Biomed. 2012, 2, 203–209. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, P.; Gill, B.S.; Kaur, S. Effect of incorporating hydrothermal, kilned and defatted oats on antioxidant and chapatti making properties of wheat flour. Food Chem. 2013, 138, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Mohan, S.; Purushothaman, D.; Jayaraj, S.; Rangarajan, A. PAL-ASE Activity in the roots of Sorghum Bicolor (L.) inoculated with Azospirillum. Curr. Sci. 1988, 57, 492–493. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Dykes, L.; Seitz, L.; Rooney, W.; Rooney, L. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 128, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Schutt, C.; Netzly, D. Effect of apiforol and apigeninidin on growth of selected fungi. J. Chem. Ecol. 1991, 17, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Food Chemistry Anthocyanins from black sorghum and their antioxidant properties. Food Chem. 2004, 90, 293–301. [Google Scholar] [CrossRef]
- Shih, C.H.; Siu, S.O.; Ng, R.; Wong, E.; Chiu, L.C.M.; Chu, I.K.; Lo, C. Quantitative analysis of anticancer 3-deoxyanthocyanidins in infected sorghum seedlings. J. Agric. Food Chem. 2007, 55, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.C.; Verdier, K.; Nicholson, R.L. Accumulation of 3-deoxyanthocyanidin phytoalexins and resistance to Colletotrichum sublineolum in sorghum. Physiol. Mol. Plant Pathol. 1999, 55, 263–273. [Google Scholar] [CrossRef]
- Seitz, L. Effects of plants-type (purple vs. tan) and mold invasion on the concentration of 3-deoxyanthocyanidins in sorghum grain. In AACC Annual Meeting Abstract; AACC International: St. Paul, MN, USA, 2004. [Google Scholar]
- Waniska, R.; Rooney, L.W. Structure and Chemistry of the Sorghum Caryopsis; John Wiley and Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Bureau of Meteorology Climate Data Online. Available online: http://www.bom.gov.au/climate/data (accessed on 10 September 2011).
- Joe White Malting System. Newmalt Micromalting Operations Manual; Joe White Maltings Pty. Limited: Adelaide, Australia, 2002. [Google Scholar]
- Kaluza, W.Z.; Mcgrath, R.M.; Roberts, T.C.; Schroder, H.H. Separation of phenolics of Sorghum bicolor (L.) Monech grain. J. Agric. Food Chem. 1980, 28, 1191–1196. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Gous, F. Tannins and Phenols in Black Sorghum. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1989. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantative methods for analysis. 2. Determination of total anthocyanin degradation index for cranberry juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Treatment | L* | a* | b* | ||
|---|---|---|---|---|---|
| Process | Region | Cultivar | |||
| Raw | 43.26 ± 2.08 a | 16.77 ± 1.21 a | 16.99 ± 1.87 a | ||
| Malted | 40.82 ± 2.08 b | 15.10 ± 1.18 b | 14.44 ± 2.12 b | ||
| Yallaroi | 43.56 ± 2.06 a | 15.96 ± 1.18 b | 17.40 ± 1.66 a | ||
| Norwin | 39.89 ± 1.44 c | 16.20 ± 1.69 a | 13.77 ± 2.10 b | ||
| Bellatta | 42.67 ± 2.47 b | 15.66 ± 1.44 c | 15.97 ± 1.76 c | ||
| G22 | 42.57 ± 1.73 c | 17.33 ± 1.12 a | 17.43 ± 2.09 a | ||
| G56 | 40.76 ± 2.00 e | 16.65 ± 1.24 b | 15.00 ± 2.43 f | ||
| G99 | 41.60 ± 1.83 d | 15.96 ± 1.23 d | 15.60 ± 2.38 d | ||
| Dominator | 41.06 ± 1.53 e | 16.46 ± 1.68 c | 15.23 ± 1.75 e | ||
| Eclipse | 40.96 ± 1.79 e | 15.60 ± 1.21 e | 14.36 ± 2.02 g | ||
| MR43 | 42.65 ± 1.89 b,c | 15.37 ± 1.45 f | 15.96 ± 1.66 c | ||
| Buster | 43.78 ± 3.64 a | 14.58 ± 0.95 g | 16.44 ± 2.90 b | ||
| Bazley | 42.95 ± 3.78 b | 15.57 ± 1.06 e | 15.70 ± 2.73 d | ||
| Cultivar | Process | L* | a* | b* |
|---|---|---|---|---|
| G22 | Raw | 43.64 ± 0.93 c | 18.26 ± 0.32 a | 18.73 ± 1.06 a |
| Malted | 41.51 ± 1.73 g,h | 16.40 ± 0.76 e | 16.12 ± 2.09 e | |
| G56 | Raw | 41.88 ± 2.11 f,g | 17.42 ± 0.59 b | 16.13 ± 2.25 e |
| Malted | 39.64 ± 1.16 j | 15.88 ± 1.26 g | 13.87 ± 2.21 j | |
| G99 | Raw | 42.79 ± 1.65 d | 15.44 ± 1.56 h | 16.81 ± 1.97 d |
| Malted | 40.41 ± 1.11 i | 16.48 ± 0.49 d | 14.40 ± 2.26 i | |
| Dominator | Raw | 42.46 ± 0.48d,e | 17.44 ± 1.73 b | 16.77 ± 0.60 d |
| Malted | 39.66 ± 0.45 j | 15.48 ± 0.97 h | 13.69 ± 0.84 k | |
| Eclipse | Raw | 42.19 ± 1.50 e,f | 16.62 ± 0.83 c | 15.89 ± 0.93 f |
| Malted | 39.74 ± 1.11 j | 14.57 ± 0.40 k | 12.84 ± 1.60 l | |
| MR43 | Raw | 43.99 ± 1.70 b,c | 16.25 ± 1.37 f | 17.35 ± 0.73 c |
| Malted | 41.31 ± 0.79 h | 14.50 ± 0.96 l | 14.57 ± 0.96 h | |
| Buster | Raw | 44.94 ± 3.75 a | 15.33 ± 0.72 i | 17.56 ± 2.60 b |
| Malted | 42.63 ± 3.43 d,e | 13.83 ± 0.32 m | 15.32 ± 2.96 g | |
| Bazley | Raw | 44.19 ± 4.15 b | 16.39 ± 0.48 e | 16.70 ± 2.76 d |
| Malted | 41.71 ± 3.24 f,g,h | 14.74 ± 0.77 j | 14.70 ± 2.54 h |
| Treatment | TPC | Tannin | Flavan-4-ols | T-Anthocyanins | T-Flavonoids | ||
|---|---|---|---|---|---|---|---|
| Process | Region | Cultivar | |||||
| Raw | 2.77 ± 0.38 a | 0.86 ± 0.26 a | 2.98 ± 0.57 a | 6.78 ± 2.26 b | 1.24 ± 0.19 a | ||
| Malted | 2.48 ± 0.25 b | 0.63 ± 0.16 b | 2.23 ± 0.60 b | 16.82 ± 6.78 a | 1.09 ± 0.15 b | ||
| Yallaroi | 2.79 ± 0.32 a | 0.78 ± 0.28 a | 2.57 ± 1.02 b | 9.18 ± 3.86 c | 1.05 ± 0.12 c | ||
| Norwin | 2.72 ± 0.30 a | 0.79 ± 0.24 a | 2.54 ± 0.62 b | 16.40 ± 9.31 a | 1.16 ± 0.16 b | ||
| Bellatta | 2.37 ± 0.29 b | 0.67 ± 0.17 b | 2.70 ± 0.13 a | 9.82 ± 4.34 b | 1.29 ± 0.20 a | ||
| G22 | 2.66 ± 0.42 b,c | 0.82 ± 0.35 a,b | 2.86 ± 0.48 a | 14.37 ± 8.42 a | 1.19 ± 0.22 c | ||
| G56 | 2.80 ± 0.28 a | 0.80 ± 0.31 a,b | 2.89 ± 0.90 a | 12.50 ± 6.41 d | 1.16 ± 0.22 d | ||
| G99 | 2.61 ± 0.41 c,d | 0.69 ± 0.19 b,c | 2.66 ± 0.87 b | 12.33 ± 6.08 d | 1.76 ± 1.15 d | ||
| Dominator | 2.49 ± 0.39 d,e | 0.75 ± 0.27 a,b | 2.56 ± 0.67 c | 10.22 ± 6.28 e | 1.16 ± 0.20 d | ||
| Eclipse | 2.77 ± 0.32 a,b | 0.84 ± 0.18 a | 2.71 ± 0.63 b | 12.93 ± 7.46 c | 1.25 ± 0.14 a | ||
| MR43 | 2.67 ± 0.28 b,c | 0.72 ± 0.20 a,b,c | 2.65 ± 0.66 b | 13.46 ± 9.92 b | 2.86 ± 1.21 b | ||
| Buster | 2.43 ± 0.35 e | 0.62 ± 0.15 c | 2.16 ± 0.56 e | 10.07 ± 6.56 e | 1.14 ± 0.25 e | ||
| Bazley | 2.60 ± 0.27 d,c | 0.72 ± 0.21 a,b,c | 2.36 ± 0.52 d | 8.52 ± 4.01 f | 1.08 ± 0.22 f | ||
| Cultivar | Process | TPC | Tannin | Flavan-4-ols | T-Anthocyanins | T-Flavonoids |
|---|---|---|---|---|---|---|
| G22 | Raw | 2.80 ± 0.58 a,b,c | 1.02 ± 0.38 a | 3.11 ± 0.58 b,c | 9.07 ± 1.83 h | 1.21 ± 0.15 e |
| Malted | 2.52 ± 0.12 e,f,g | 0.61 ± 0.16 d,e | 2.61 ± 0.13 e,f | 19.66 ± 9.23 b | 1.18 ± 0.29 f,g | |
| G56 | Raw | 2.85 ± 0.33 a,b | 0.91 ± 0.38 a,b | 3.27 ± 1.01 a | 7.16 ± 1.43 k,j | 1.22 ± 0.18 d |
| Malted | 2.75 ± 0.23 b,c,d | 0.68 ± 0.21 c,d,e | 2.52 ± 0.63 f,g | 17.84 ± 4.47 c | 1.09 ± 0.09 i | |
| G99 | Raw | 2.84 ± 0.45 a,b | 0.83 ± 0.19 b,c | 3.22 ± 0.83 a,b | 7.50 ± 1.71 j | 1.22 ± 0.16 d,e |
| Malted | 2.37 ± 0.19 g,h | 0.55 ± 0.01 e | 2.09 ± 0.48 i | 17.16 ± 4.73 d | 1.09 ± 0.06 i | |
| Dominator | Raw | 2.59 ± 0.46 d,e,f | 0.90 ± 0.30 a,b | 3.05 ± 0.35 c | 5.80 ± 1.76 l | 1.25 ± 0.22 c |
| Malted | 2.40 ± 0.33 g | 0.61 ± 0.16 d,e | 2.07 ± 0.54 i | 14.65 ± 6.07 f | 1.07 ± 0.15 j | |
| Eclipse | Raw | 2.97 ± 0.35 a | 0.90 ± 0.16 a,b | 3.10 ± 0.36 b,c | 7.98 ± 3.04 i | 1.32 ± 0.11 a |
| Malted | 2.58 ± 0.15 d,e,f | 0.77 ± 0.20 b,c,d | 2.32 ± 0.62 h | 17.89 ± 7.37 c | 1.19 ± 0.15 f | |
| MR43 | Raw | 2.75 ± 0.34 b,c,d | 0.76 ± 0.21 b,c,d | 2.87 ± 0.33 d | 6.75 ± 2.91 k | 1.29 ± 0.16 b |
| Malted | 2.59 ± 0.20 d,e,f | 0.68 ± 0.21 c,d,e | 2.41 ± 0.86 g,h | 20.18 ± 10.00 a | 1.13 ± 0.17 h | |
| Buster | Raw | 2.65 ± 0.34 c,d,e | 0.69 ± 0.20 c,d,e | 2.50 ± 0.18 f,g | 4.78 ± 1.06 m | 1.29 ± 0.27 b |
| Malted | 2.21 ± 0.19 h | 0.55 ± 0.00 e | 1.82 ± 0.61 j | 15.35 ± 5.15 e | 0.98 ± 0.08 l | |
| Bazley | Raw | 2.75 ± 0.23 b,c,d | 0.83 ± 0.21 b,c | 2.70 ± 0.14 e | 5.22 ± 0.39 m | 1.17 ± 0.30 g |
| Malted | 2.45 ± 0.23 g,f | 0.61 ± 0.17 d,e | 2.03 ± 0.56 i | 11.82 ± 3.04 g | 1.00 ± 0.03 k |
| Treatment | Luteolinidin | Apigeninidin | 5-Methoxy-luteolinidin | 7-Methoxy-apigeninidin | ||
|---|---|---|---|---|---|---|
| Process | Region | Cultivar | ||||
| Raw | 4.28 ± 4.24 b | 4.43 ± 3.20 b | 4.35 ± 4.19 b | 5.95 ± 5.34 b | ||
| Malted | 43.93 ± 33.13 a | 37.89 ± 12.27 a | 25.71 ± 23.08 a | 46.09 ± 19.78 a | ||
| Yallaroi | 12.58 ± 11.95 c | 20.05 ± 18.91 b | 5.71 ± 4.59 c | 20.42 ± 19.73 b | ||
| Norwin | 45.16 ± 42.88 a | 25.73 ± 20.69 a | 31.99 ± 25.95 a | 39.41 ± 30.78 a | ||
| Bellatta | 14.58 ± 15.44 b | 17.69 ± 17.01 c | 7.39 ± 6.74 b | 18.22 ± 16.32 c | ||
| G22 | 28.09 ± 33.06 b | 14.87 ± 12.36 e | 20.14 ± 23.12 a | 22.47 ± 22.57 c | ||
| G56 | 23.31 ± 22.84 c,d | 24.27 ± 24.44 a,b | 13.20 ± 16.94 d,e | 24.04 ± 29.28 c | ||
| G99 | 24.27 ± 22.40 c | 22.61 ± 20.67 c | 16.07 ± 16.00 c | 30.15 ± 30.58 a | ||
| Dominator | 22.00 ± 29.73 d | 19.08 ± 17.47 d | 14.18 ± 20.07 d | 23.37 ± 22.49 c | ||
| Eclipse | 30.50 ± 46.95 a | 22.09 ± 18.98 c | 17.05 ± 25.81 c | 27.16 ± 24.73 b | ||
| MR43 | 30.48 ± 43.66 a | 22.70 ± 17.37 b,c | 18.20 ± 26.41 b | 29.83 ± 23.36 a | ||
| Buster | 17.88 ± 21.11 e | 25.32 ± 25.25 a | 12.28 ± 16.64 e | 30.24 ± 8.90 a | ||
| Bazley | 16.32 ± 20.48 e | 18.32 ± 15.97 d | 9.14 ± 11.37 f | 20.89 ± 16.84 d | ||
| Cultivar | Process | Luteolinidin | Apigeninidin | 5-Methoxyluteolinidin | 7-Methoxyapigeninidin |
|---|---|---|---|---|---|
| G22 | Raw | 6.62 ± 6.07 g | 3.90 ± 3.98 f,g | 8.12 ± 6.55 f | 6.36 ± 6.38 h |
| Malted | 49.56 ± 35.53 b | 25.85 ± 5.56 d | 32.16 ± 28.04 a | 38.57 ± 21.40 e | |
| G56 | Raw | 3.65 ± 0.60 h,i | 3.79 ± 3.03 f,g | 2.94 ± 0.73 j,h,i | 2.99 ± 2.02 j |
| Malted | 42.97 ± 14.81 c,d | 44.74 ± 17.28 a | 23.46 ± 19.44 c | 45.08 ± 28.62 d | |
| G99 | Raw | 3.67 ± 1.55 h,i | 5.41 ± 5.99 e,f | 3.98 ± 2.57 g,h,i | 8.74 ± 10.68 g |
| Malted | 44.86 ± 9.16 c | 39.80 ± 13.97 b | 28.17 ± 14.34 b | 51.55 ± 29.05 b | |
| Dominator | Raw | 3.63 ± 2.94 h,i | 2.87 ± 0.85 g | 4.23 ± 3.37 g,h | 3.60 ± 1.22 I,j |
| Malted | 40.37 ± 33.56 d | 35.30 ± 6.28 c | 24.12 ± 25.24 c | 43.13 ± 13.17 d | |
| Eclipse | Raw | 5.70 ± 6.77 g,h | 4.72 ± 2.04 e,f,g | 5.34 ± 6.11 g | 5.83 ± 2.27 h |
| Malted | 55.30 ± 57.68 a | 39.46 ± 8.02 b | 28.77 ± 33.14 b | 48.48 ± 15.68 c | |
| MR43 | Raw | 6.21 ± 6.92 g,h | 6.47 ± 3.05 e | 5.22 ± 5.48 g | 8.84 ± 6.07 g |
| Malted | 54.76 ± 52.26 a | 38.93 ± 4.69 b | 31.20 ± 33.18 a | 50.82 ± 10.34 b | |
| Buster | Raw | 2.49 ± 1.76 i | 4.68 ± 2.42 e,f,g | 2.77 ± 2.30 i,j | 5.62 ± 3.95 h,i |
| Malted | 33.28 ± 20.23 e | 45.95 ± 19.39 a | 21.78 ± 19.68 d | 54.86 ± 24.94 a | |
| Bazley | Raw | 2.27 ± 0.88 i | 3.57 ± 2.11 f,g | 2.25 ± 0.99 j | 5.57 ± 3.31 h,i |
| Malted | 30.36 ± 21.18 f | 33.06 ± 5.93 c | 16.03 ± 13.01 e | 36.20 ± 7.08 f |
| Region | Mean Temperature (°C) | Precipitation (mm) | |
|---|---|---|---|
| Min | Max | ||
| Norwin | 18 | 29.8 | 34.3 |
| Yallaroi | 21 | 34.5 | 44.2 |
| Bellata | 20.8 | 34.5 | 57.4 |
| Stage | Stage | Temperature (°C) | Time (h) |
|---|---|---|---|
| Steeping | Water | 17 | 8:00 |
| Air rest | 17 | 10:00 | |
| Water | 17 | 2:00 | |
| Germination | Water bed | 17 | 96:00 |
| Kilning | Air | 50 | 6:00 |
| 55 | 2:00 | ||
| 60 | 2:00 | ||
| 65 | 1:30 | ||
| 70 | 1:30 | ||
| 75 | 3:30 | ||
| 85 | 3:30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoddami, A.; Mohammadrezaei, M.; Roberts, T.H. Effects of Sorghum Malting on Colour, Major Classes of Phenolics and Individual Anthocyanins. Molecules 2017, 22, 1713. https://doi.org/10.3390/molecules22101713
Khoddami A, Mohammadrezaei M, Roberts TH. Effects of Sorghum Malting on Colour, Major Classes of Phenolics and Individual Anthocyanins. Molecules. 2017; 22(10):1713. https://doi.org/10.3390/molecules22101713
Chicago/Turabian StyleKhoddami, Ali, Mohammad Mohammadrezaei, and Thomas H. Roberts. 2017. "Effects of Sorghum Malting on Colour, Major Classes of Phenolics and Individual Anthocyanins" Molecules 22, no. 10: 1713. https://doi.org/10.3390/molecules22101713