A Highly Selective and Sensitive Fluorescent Turn-Off Probe for Cu2+ Based on a Guanidine Derivative
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Spectral Characteristics
2.3. Calculation of Binding Constants and Different Concentrations of Cu2+
2.4. Response Time
2.5. Effect of pH
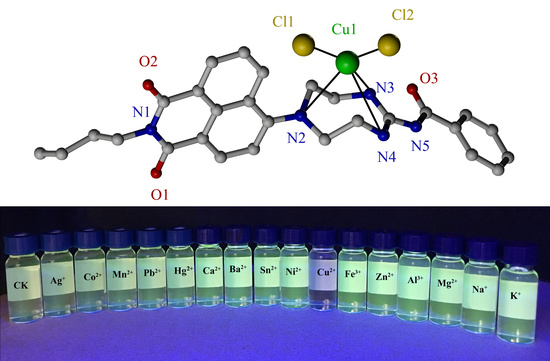
2.6. Selectivity
2.7. Thermal Studies
2.8. IR Spectra
3. Experimental
3.1. Chemicals and Instruments
3.2. Testing Methods
3.3. Synthesis of N-n-Butyl-4-bromo-1,8-naphthalimide (1)
3.4. Synthesis of N-n-Butyl-4-N′,N′-dihydroxyethyl-1,8-naphthalimide (2)
3.5. Synthesis of N-n-Butyl-4-N′,N′-diaminoethyl-1,8-naphthalimide (4)
3.6. Synthesis of N-n-Butyl-4-(1′-cyclooctene-1′,3′,6′-triazole)-1,8-naphthalimide (L)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Manez, R.; Sancenon, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.Q.; Kim, J.S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Tang, Y.Y.; Zhu, W.H.; Xie, Y.S. Fluorescent and colorimetric ion probes based on conjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef] [PubMed]
- Thiele, D.J.; Gitlin, A.D. Assembling the pieces. Chem. Biol. 2008, 4, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Pradhan, T.; Lee, Y.M.; Kim, J.S.; Kim, S. A calix[2] Triazole[2] Arene-based fluorescent chemosensor for probing the copper trafficking pathway in Wilson’s disease. Dalton Trans. 2014, 43, 16178–16182. [Google Scholar] [CrossRef] [PubMed]
- You, G.R.; Park, G.J.; Lee, J.J.; Kim, C. A colorimetric sensor for the sequential detection of Cu2+ and CN− in fully aqueous media: Practical performance of Cu2+. Dalton Trans. 2015, 44, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mare, S.; Penugonda, S.; Robinson, S.M.; Dohgu, S.; Banks, W.A.; Ercal, N. Copper complexing decreases the ability of amyloid beta peptide to cross the BBB and enter brain parenchyma. Peptides 2007, 28, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Gray, H.B.; Winkler, J.R. Copper(II) binding to alpha-synuclein, the Parkinson’s protein. J. Am. Chem. Soc. 2008, 130, 6898–6899. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Hu, W.T.; Gao, H.F.; Qi, H.L.; Diang, L.P. Luminescence of ferrocene-modified pyrene derivatives for turn-on sensing of Cu2+ and anions. Spectochim. Acta A 2017, 184, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.H.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+ -selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Xu, J.; Yang, F.; Zhou, W.; Li, Z.X.; Wei, L.H.; Yu, M.M. Nanomolar Cu2+ and F− naked-eye detection with a 1,8-naphthalimide-based colorimetric probe. Sens. Actuators B 2015, 212, 364–370. [Google Scholar] [CrossRef]
- Khan, B.; Shah, M.R.; Ahmed, D.; Rabnawaz, M.; Anis, I.; Afridi, S.; Makhmoor, T.; Tahir, M.N. Synthesis, characterization and Cu2+ triggered selective fluorescence quenching of bis-calix[4]arene tetra-triazole macrocycle. J. Hazard. Mater. 2016, 309, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; Merlin, C.; Dezanet, C.; Balan, L.; Medjandi, G.; Ben-Attia, M.; Schneider, R. Trace amounts of Cu2+ ions influence ROS production and cytotoxicity of ZnO quantum dots. J. Hazard. Mater. 2016, 304, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Hajishabani, A.; Dadfarnia, S.; Zamani, H. Use of clinoptilolite loaded with 1-(2-pyridylazo)-2-naphthol as a sorbent for preconcentration of Pb(II), Ni(II), Cd(II) and Cu(II) prior to their determination by flame atomic absorption spectroscopy. Int. J. Environ. Anal. Chem. 2009, 89, 223–231. [Google Scholar] [CrossRef]
- Tomalova, I.; Foltynova, P.; Kanicky, V.; Preisler, J. MALDI MS and ICP MS detection of a single CE separation record: A tool for metalloproteomics. Anal. Chem. 2014, 86, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.H.; Mao, Z.G.; Qing, T.P.; He, X.X.; Zou, Z.; He, D.G.; Shi, H.; Huang, J.; Liu, J.B.; Wang, K.M. Visual and portable strategy for copper(II) detection based on a striplike poly(thymine)-caged and microwell-printed hydrogel. Anal. Chem. 2014, 86, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Jia, X.L.; Bian, P.P.; Ma, Z.F. A simple and novel system for colorimetric detection of cobalt ions. Analyst 2014, 139, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Niu, J.Y.; Yu, C.G.; Zhuo, L.H.; Ge, J.C. Highly luminescent water-soluble Cd Te nanowires as fluorescent probe to detect copper(II). Chem. Commun. 2005, 33, 4184–4186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Q.; Su, Y.Y.; Li, J.; Li, D.; Zhang, J.; Song, S.P.; Zhao, Y.; Li, G.X.; Fan, C.H. Highly sensitive electrochemical sensor for mercury(II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based amplification. Anal. Chem. 2009, 81, 7660–7666. [Google Scholar] [CrossRef] [PubMed]
- Niamnont, N.; Khumsri, A.; Promchat, A.; Tumcharern, G.; Sukwattanasinitt, M. Novel salicylaldehyde derivatives as fluorescence turn-on sensors for cyanide ion. J. Hazard. Mater. 2014, 280, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Zeng, A.L.; Luo, H.Q.; Li, N.B. Fluorescent silver nanoclusters for ultrasensitive determination of chromium(VI) in aqueous solution. J. Hazard. Mater. 2016, 304, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Czamik, A.W. Fluorescent chemosensors for ion and molecule recognition. In ACS Symposium Series Sponsored by the Division of Organic Chemistry of the American Chemical Society at the 204th Meeting of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1992; p. 538. [Google Scholar]
- Dujols, V.; Ford, F.; Czarnik, A.W. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J. Am. Chem. Soc. 1997, 119, 7386–7387. [Google Scholar] [CrossRef]
- Xiang, Y.; Tong, A.J.; Jin, P.Y.; Ju, Y. New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org. Lett. 2006, 8, 2863–2866. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Zhu, W.H.; Xie, Y.S. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef] [PubMed]
- Sirilaksanapong, S.; Sukwattanasinitt, M.; Rashatasakhon, P. 1,3,5-Triphenylbenzene fluorophore as a selective Cu2+ sensor in aqueous media. Chem. Commun. 2012, 48, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, S.; Tabakci, B.; Tabakci, M. A highly selective fluorescent sensor based on calix[4]arene appended benzothiazole units for Cu2+, S2− and HSO4− ions in aqueous solution. Sens. Actuators B Chem. 2016, 228, 109–116. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, Q.; Yan, C.; Xu, C.H.; Huang, X.H.; Hu, S.L. A new fluorescence chemosensor for selective detection of copper ion in aqueous solution. Tetrahedron Lett. 2016, 57, 2239–2243. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, J.; Xie, K.F.; Xi, P.X.; Hou, F.P.; Li, Z.P.; Xie, G.Q.; Shi, Y.J.; Liu, H.Y.; Bai, D.C.; et al. Cu2+-selective fluorescent chemosensor based on coumarin and its application in bioimaging. Dalton Trans. 2011, 40, 10815–10817. [Google Scholar] [CrossRef] [PubMed]
- Maher, N.J.; Diao, H.W.; O’Sullivan, J.; Fadda, E.; Heaney, F.; McGinley, J. Lower rim isoxazole-calix[4]arene derivatives as fluorescence sensors for copper(II) ions. Tetrahedron 2015, 71, 9223–9233. [Google Scholar] [CrossRef]
- Ganguly, A.; Ghosh, S.; Kar, S.; Guchhait, N. Selective fluorescence sensing of Cu(II) and Zn(II) using a simple Schiff base ligand: Naked eye detection and elucidation of photoinduced electron transfer (PET) mechanism. Spectrochim. Acta Part A 2015, 143, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Sun, T.T.; Lin, W.H.; Guan, X.G.; Zheng, M.; Xie, Z.G.; Jing, X.B. Bodipy fluorescent chemosensor for Cu2+ detection and its applications in living cells: Fast response and high sensitivity. J. Fluoresc. 2014, 24, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Poss, M.A.; Iwanowicz, E.; Reid, J.A.; Lin, J.; Gu, Z.X. A mild and efficient method for the preparation of guanidines. Tetrahedron Lett. 1992, 33, 5933–5936. [Google Scholar] [CrossRef]
- Liu, B.; Tian, H. A selective fluorescent ratiometric chemodosimeter for mercury ion. Chem. Commun. 2005, 3156–3158. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Georges, J. Fluorescence quantum yield of rhodamine 6G in ethanol as a function of concentration using thermal lens spectrometry. Chem. Phys. Lett. 1996, 260, 115–118. [Google Scholar] [CrossRef]
- Ma, Q.J.; Zhang, X.B.; Han, Z.X.; Huang, B.; Jiang, Q.; Shen, G.L.; Yu, R.Q. A ratiometric fluorescent probe for zinc ions based on the quinoline fluorophore. Int. J. Environ. Anal. Chem. 2011, 91, 74–86. [Google Scholar] [CrossRef]
- Gao, C.J.; Liu, X.; Jin, X.J.; Wu, J.; Xie, Y.J.; Liu, W.S.; Yao, X.J.; Tang, Y. A retrievable and highly selective fluorescent sensor for detecting copper and sulfide. Sens. Actuators B Chem. 2013, 185, 125–131. [Google Scholar] [CrossRef]
- Karak, D.; Banerjee, A.; Sahana, A.; Guha, S.; Lohar, S.; Adhikari1, S.S.; Das, D. 9-Acridone-4-carboxylic acid as an efficient Cr(III) fluorescent sensor: Trace level detection, estimation and speciation studies. J. Hazard. Mater. 2011, 188, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.S.; Ding, Y.B.; Li, X.; Wang, C.; Hill, J.P.; Ariga, K.; Zhang, W.B.; Zhu, W.H. Selective, sensitive and reversible “turn-on” fluorescent cyanide probes based on 2,2′-dipyridylaminoanthracene-Cu2+ ensembles. Chem. Commun. 2012, 48, 11513–11515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yang, L.; Zhang, W.B.; Zhou, Y.; Zhao, B.; Li, X.Y. A colorimetric probe for copper(II) ion based on 4-amino-1,8-naphthalimide. Inorg. Chim. Acta 2012, 381, 111–116. [Google Scholar] [CrossRef]
- Gao, Y.G.; Tang, Q.; Shi, Y.D.; Zhang, Y.; Lu, Z.L. 1,8-naphthalimide modified [12]aneN compounds as selective and sensitive probes for Cu2+ ions and ATP in aqueous solution and living cells. Talanta 2016, 152, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Huo, F.J.; Liu, T.; Wen, Y.; Yin, C.X. A rapid and highly sensitive fluorescent imaging materials for thiophenols. Dyes Pigm. 2016, 133, 248–254. [Google Scholar] [CrossRef]
- Guo, X.F.; Zhu, B.C.; Liu, Y.Y.; Zhang, Y.; Jia, L.H.; Qian, X.H. Synthesis and properties of N-butyl-4-(aza-15-crown-5)-1, 8-naphthalimide as a fluorescent probe. Chin. J. Org. Chem. 2006, 26, 504–507. [Google Scholar]
- Lee, S.; Lee, J.H.; Pradhan, T.; Lim, C.S.; Cho, B.R.; Bhuniya, S.; Kim, S.; Kim, J.S. Fluorescent turn-on Zn2+ sensing in aqueous and cellular media. Sens. Actuators B Chem 2011, 160, 1489–1493. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, K.H.; Liu, H.G.; Park, S.Y.; Yoon, S.S.; Lee, J.Y.; Kim, Y.G. A bis(pyridine-2-ylmethyl)amine-based selective and sensitive colorimetric and fluorescent chemosensor for Cu2+. Sens. Actuators B Chem. 2016, 222, 28–34. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors’ lab. |















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Chai, Q.; Liang, X.-M.; Li, M.-Q.; Wang, Z.-Q.; Fu, Y. A Highly Selective and Sensitive Fluorescent Turn-Off Probe for Cu2+ Based on a Guanidine Derivative. Molecules 2017, 22, 1741. https://doi.org/10.3390/molecules22101741
Ye F, Chai Q, Liang X-M, Li M-Q, Wang Z-Q, Fu Y. A Highly Selective and Sensitive Fluorescent Turn-Off Probe for Cu2+ Based on a Guanidine Derivative. Molecules. 2017; 22(10):1741. https://doi.org/10.3390/molecules22101741
Chicago/Turabian StyleYe, Fei, Qiong Chai, Xiao-Min Liang, Ming-Qiang Li, Zhi-Qiang Wang, and Ying Fu. 2017. "A Highly Selective and Sensitive Fluorescent Turn-Off Probe for Cu2+ Based on a Guanidine Derivative" Molecules 22, no. 10: 1741. https://doi.org/10.3390/molecules22101741