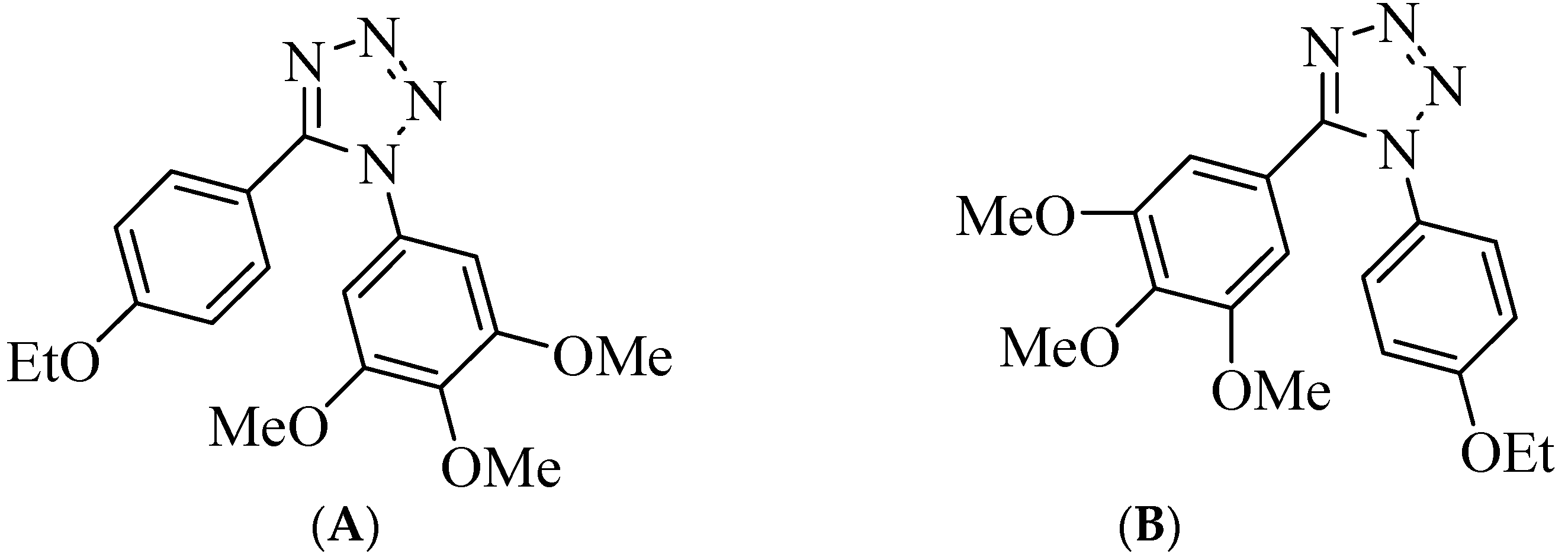3. Experimental
Melting points were recorded on a Thermocouple digital melting point apparatus (Stuart, Staffordshire, UK) and are uncorrected. IR spectra were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA) with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, Merck kieselgel 60 (0.063–0.200 mm) (Merck KGaA, Frankfurt, Germany) was used as the stationary phase. NMR spectra were obtained as DMSO-
d6 solutions using either the Varian 300 MHz NMR spectrometer (Varian Inc., Palo Alto, CA, USA) or Agilent 500 MHz NMR spectrometer (Agilent Technologies, Oxford, UK) and the chemical shifts are quoted relative to the TMS peak. Low- and high-resolution mass spectra were recorded at an ionization potential of 70 eV using Waters Synapt G2 Quadrupole Time-of-flight mass spectrometer (Waters Corp., Milford, MA, USA) at the University of Stellenbosch Mass Spectrometry Unit. The elemental analyses were obtained using a Vario EL Cube Elemental Analyzer (Elementar, Langenselbold, Germany) at the University of Stellenbosch Central Analytical Facility. The synthesis and characterization of compounds
1a–
c have been described before [
7].
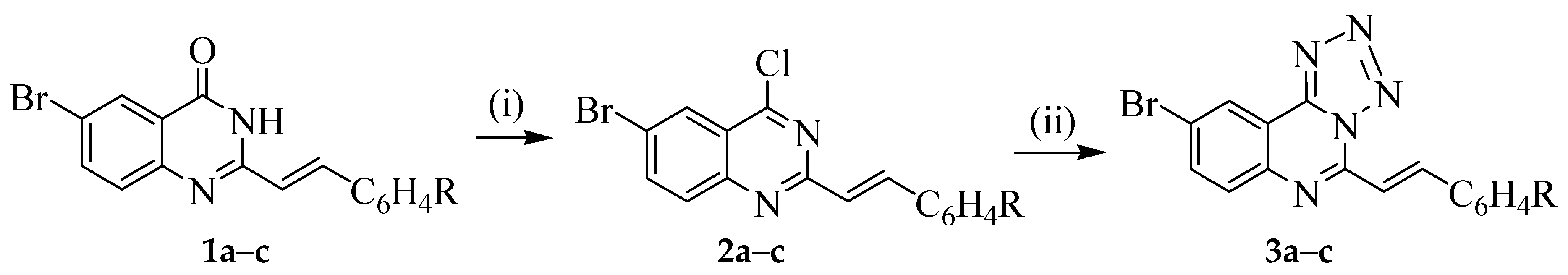
3.1. Typical Procedure for the Phosphoryl-Mediated Chlorination-Dehydration of 1a–c; the Synthesis of 1a–c
A mixture of 6-bromo-2-styrylquinazolin-4(3H)-one 1a (0.50 g, 1.55 mmol), phosphoryl chloride (20 mL) and triethylamine (2 drops) was heated under reflux for 8 h and then allowed to cool to RT. The mixture was poured slowly into an ice-cold aqueous ammonia with vigorous stirring. The resulting precipitate was filtered, washed thoroughly with ice-cold water and then recrystallized to afford 2. Compounds 2a–c were prepared in this fashion.
(E)-6-Bromo-4-chloro-2-styrylquinazoline (2a). Yellow solid (0.41 g, 78%), m.p. 203–205 °C (ethanol); νmax (ATR) 539, 682, 752, 839, 972, 1557, 3934 cm−1; 1H-NMR (500 MHz, DMSO-d6) 7.27 (1H, d, Jtrans = 16.0 Hz, Hb), 7.36–7.44 (3H, m, 3′,5′-H and 4′-H), 7.65 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.85 (1H, d, J = 8.7 Hz, 8-H), 7.98 (1H, dd, J = 2.4 Hz and 8.7 Hz, 7-H), 8.13 (1H, d, Jtrans = 16.0 Hz, Ha), 8.35 (1H, d, J = 2.4 Hz, 5-H); 13C-NMR (125 MHz, DMSO-d6) 119.3, 120.2, 122.9, 128.3, 128.6, 129.0, 129.6, 130.7, 135.1, 138.0, 140.4, 147.1, 153.0, 161.0; HRMS (ES): MH+, found 344.9785. C16H11N235Cl79Br+ requires 344.9794. Anal. calcd. for C16H10N2ClBr: C, 55.60; H, 2.92; N, 8.11. Found: C, 55.63; H, 2.90; N, 8.06.
(E)-6-Bromo-4-chloro-2-(4-fluorostyryl)quinazoline (2b). Yellow solid (0.37 g, 71%), m.p. 215–217 °C (ethanol); νmax (ATR) 520, 819, 828, 948, 1208, 1598, 2922 cm−1; 1H-NMR (300 MHz, DMSO-d6) 7.10 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.20 (1H, d, Jtrans = 16.0 Hz, Hb), 7.63 (2H, dd, J = 2.0 Hz and 9.0 Hz, 2′,6′-H), 7.85 (1H, d, J = 8.7 Hz, 8-H), 8.00 (1H, dd, J = 2.4 Hz and 8.7 Hz, 7-H), 8.10 (1H, d, Jtrans = 16.0 Hz, Ha), 8.37 (1H, d, J = 2.4 Hz, 5-H); 13C-NMR (75 MHz, DMSO-d6) 166.6 (d, 2JCF = 20.9 Hz), 119.2, 120.4, 123.0, 128.5, 129.2, 130.45 (d, 3JCF = 8.5 Hz), 131.8 (d, 4JCF = 3.8 Hz), 137.9, 138.8, 147.5, 152.6, 160.9, 163.5 (d, 1JCF = 247.5 Hz); HRMS (ES): MH+, found 362.9698. C16H10N2F35Cl79Br+ requires 362.9700. Anal. calcd. for C16H9N2FClBr: C, 52.85; H, 2.49; N, 7.70. Found: C, 52.83; H, 2.47; N, 7.68.
(E)-6-Bromo-4-chloro-2-(4-chlorostyryl)quinazoline (2c). Yellow solid (0.47 g, 71%), m.p. 210–212 °C (ethanol); νmax (ATR) 496, 662, 813, 830, 972, 991, 1558, 2921 cm−1; 1H-NMR (300 MHz, DMSO-d6) 7.24 (1H, d, Jtrans = 16.0 Hz, Hb), 7.38 (2H, d, J = 8.0 Hz, 3′,5′-H), 7.58 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.85 (1H, d, J = 9.0 Hz, 8-H), 7.99 (1H, dd, J = 2.4 Hz and 9.0 Hz, 7-H), 8.08 (1H, d, Jtrans = 16.0 Hz, Ha), 8.37 (1H, d, J = 2.4 Hz, 5-H); 13C-NMR (125 MHz, DMSO-d6) 119.3, 123.3, 124.4, 128.0, 128.3, 128.4, 130.0, 130.6, 131.7, 133.2, 133.9, 134.4, 137.8, 148.2; HRMS (ES): MH+, found: 378.9492. C16H10N235Cl79Br+ requires: 378.9404. Anal. calcd. for C16H9N2Cl2Br: C, 50.56; H, 2.39; N, 7.37. Found: C, 50.54; H, 2.36; N, 7.36.
3.2. Typical Procedure for the Synthesis of Tetrazoloquinazolines 3a–c
A stirred mixture of 2 (1 equivalent) and sodium azide (1.2 equivalent) in DMF (26 mL/mmol of 2) was heated at 45 °C for 4 h. The mixture was allowed to cool to RT and then quenched with ice-cold water. The resultant precipitate was filtered and recrystallized from acetonitrile to afford 3. The following compounds were prepared in this fashion:
(E)-9-Bromo-5-styryltetrazolo[1,5-c]quinazoline (3a). A mixture of 2a (0.20 g, 0.58 mmol) and sodium azide (0.04 g, 0.62 mmol) in DMF (15 mL) afforded 3a as a brown solid (0.15 g, 72%), m.p. 214–216 °C (acetonitrile); νmax (ATR) 558, 686, 761, 836, 975, 1076, 1385, 1495, 1633, 3026, 3082 cm−1; 1H-NMR (300 MHz, DMSO-d6) 7.48–7.50 (3H, m, 3′,5′-H and 4′-H), 7.89 (2H, d, J = 8.4 Hz, 2′,6′-H), 7.91 (1H, d, Jtrans = 16.0 Hz, Hb), 8.06 (1H, d, J = 8.7 Hz, 7-H ), 8.19 (1H, dd, J = 2.4 and 9.0 Hz, 8-H), 8.37 (1H, d, Jtrans = 16.0 Hz, Ha), 8.67 (1H, d, J = 2.4 Hz, 10-H); 13C-NMR (75 MHz, DMSO-d6) 116.5, 116.9, 122.3, 127.0, 129.1, 129.7, 131.0, 131.3, 135.0, 137.1, 142.2, 143.0, 143.6, 149.0; HRMS (ES): MH+, found: 352.0189. C16H11N579Br+ requires: 352.0188. Anal. calcd. for C16H10N5Br: C, 54.56; H, 2.86; N, 19.89. Found: C, 54.54; H, 2.89, N, 9.86.
(E)-9-Bromo-5-(4-fluorostyryl)tetrazolo[1,5-c]quinazoline (3b). A mixture of 2b (0.20 g, 0.55 mmol) and sodium azide (0.04 g, 0.66 mmol) in DMF (14 mL) afforded 3b as a brown solid (0.16 g, 81%), m.p. 217–218 °C (acetonitrile); νmax (ATR) 517, 828, 979, 1079, 1161, 123, 1269, 1470, 1508, 1586, 1637 cm−1; 1H-NMR (300 MHz, DMSO-d6) 7.34 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.89 (1H, d, Jtrans = 16.0 Hz, Hb), 8.00–8.07 (3H, m, Ar), 8.19 (1H, dd, J = 2.4 and 9.0 Hz, 8-H), 8.38 (1H, d, Jtrans = 16.0 Hz, Ha), 8.69 (1H, d, J = 2.4 Hz, 10-H); 13C-NMR (75 MHz, DMSO-d6) 116.4, 166.6 (d, 2JCF = 21.7 Hz), 116.7, 122.2, 126.7, 130.7, 131.5 (d, 3JCF = 8.5 Hz), 131.67 (d, 4JCF = 2.8 Hz), 137.2, 141.8, 142.2, 143.6, 148.7, 163.9 (d, 1JCF = 247.4 Hz); HRMS (ES): MH+, found: 370.0101. C16H10N5F79Br+ requires: 370.0104. Anal. calcd. for C16H9N5FBr: C, 51.91; H, 2.45; N, 18.92. Found: C, 51.90; H, 2.42; N, 18.89.
(E)-9-Bromo-5-(4-chlorostyryl)tetrazolo[1,5-c]quinazoline (3c). A mixture of 2c (0.20 g, 0.63 mmol) and sodium azide (0.05 g, 0.76 mmol) in DMF (20 mL) afforded 3c as a brown solid (0.15 g, 63%), m.p. 234–236 °C (acetonitrile); νmax (ATR) 465, 500, 766, 809, 820, 1090, 1387, 1470, 1501, 1582, 1633 cm−1; 1H-NMR (300 MHz, DMSO-d6) 7.44 (2H, d, J = 8.0 Hz, 3′,5′-H), 7.93 (1H, d, Jtrans = 16.0 Hz, Hb), 7.91 (2H, d, J = 7.5 Hz, 2′,6′-H), 8.05 (1H, d, J = 8.0 Hz, 7-H), 8.17 (1H, d, J = 7.5 Hz, 8-H), 8.36 (1H, d, Jtrans = 16.0 Hz, Ha), 8.70 (1H, d, J = 2.4 Hz, 10-H); 13C-NMR (75 MHz, DMSO-d6) 116.7, 117.3, 122.4, 126.7, 129.7 (2 × C), 130.8, 133.9, 135.8, 137.1, 141.5, 142.2, 143.5, 148.9; RMS (ES): MH+, found: 386.1464. C16H9N535Cl79Br + requires: 386.1464. Anal. calcd. for C16H9N5ClBr: C, 49.70; H, 2.35; N, 18.11. Found: C, 49.67; H, 2.33; N, 18.10.
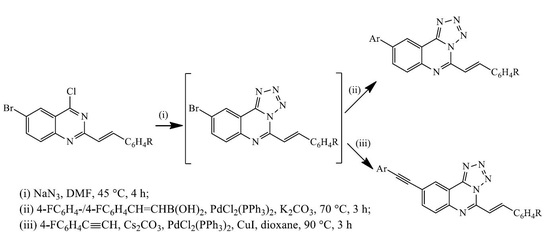
3.3. Typical Procedure for the Suzuki-Miyaura Cross-Coupling of 3a–c; Synthesis of 4a–f
To a three-necked flask equipped with stirrer, rubber septum, thermometer and a condenser fitted with a balloon at the top was added 3a (0.30 g, 0.85 mmol) and DMF (15 mL) under nitrogen atmosphere. 4-Fluorophenylboronic acid (0.18 g, 1.28 mmol), PdCl2(P(Cy)3)2 (0.06 g, 0.085 mmol), K2CO3 (0.14 g, 1.02 mmol) and water (5 mL) were added to the mixture under nitrogen atmosphere. The mixture was heated at 100 °C for 3 h and then poured into ice-cold water. The product was extracted into chloroform and the combined organic phases were washed with brine, dried over MgSO4 and the salt was filtered off. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel using 9:1 toluene-ethyl acetate (v/v) as an eluent. The following products were prepared in this fashion.
(E)-9-(4-Fluorophenyl)-5-styryltetrazolo[1,5-c]quinazoline (4a). Solid (0.17 g, 53%), Rf 0.62, m.p. 243–245 °C; νmax (ATR) 524, 588, 683, 749, 823, 972, 1227, 1484, 1634, 2854, 2924, 3029 cm−1; δH (500 MHz, DMSO-d6) 7.37 (3H, t, J = 9.0 Hz, 2″,6″-H), 7.47–7.51 (3H, m, Ph), 7.90–7.99 (5H, m, Ha, 2″,6″-H and Ph), 8.18 (1H, d, J = 9 Hz, 7-H), 8.32 (1H, dd, J = 2.0 Hz and 8.0 Hz, 8-H), 8.37 (1H, d, Jtrans = 16.0 Hz, Hb), 8.73 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 115.6, 116.6 (2JCF = 21.9 Hz), 116.8, 121.7, 129.0, 129.4, 129.7 (2 × C), 129.9 (3JCF = 8.5 Hz), 131.2, 132.7, 135.1, 140.0, 142,4, 142.5 (3JCF = 2.9 Hz), 143.1, 149.6, 163.0 (1JCF = 244.6 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.08 (1F, ddd, J = 8.0, 14.6, 21.2 Hz); HRMS (ES): MH+, found: 368.1311. C22H14N5F+ requires: 368.1307. Anal. calcd. for C16H14N5F: C, 71.92; H, 3.84; N, 19.06. Found: C, 71.91; H, 3.82; N, 19.03.
(E)-9-(4-Fluorophenyl)-5-(4-fluorostyryl)tetrazolo[1,5-c]quinazoline (4b). Solid (0.22 g, 51%), Rf 0.84, m.p. 218–220 °C; νmax (ATR) 537, 820, 975, 1158, 1234, 1484, 1511, 1635, 2853, 2924, 3045 cm−1; δH (500 MHz, DMSO-d6) 7.33 (2H, t, J = 7.5 Hz, 3′,5′-H), 7.37 (2H, t, J = 7.5 Hz, 3″,5″-H), 7.88 (1H, d, Jtrans = 16.0 Hz, Hb), 7.96 (2H, t, J = 7.5 Hz, 2′,4′-H), 8.00 (2H, t, J = 7.5 Hz, 2″,6″-H), 8.17 (1H, d, J = 8.5 Hz, 7-H), 8.32 (1H, dd, J = 2 and 8.5 Hz, 8-H), 8.36 (1H, d, Jtrans = 16.0 Hz, Ha), 8.71 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 115.6, 116.6 (2JCF = 21.8 Hz), 116.7 (2JCF = 21.8 Hz), 116.8, 121.7, 129.4, 129.9 (3JCF = 8.5 Hz), 131.4 (3JCF = 8.5 Hz), 131.8 (3JCF = 2.8 Hz), 132.6, 135.0 (3JCF = 2.9 Hz), 140.0, 141.3, 142.5 (3JCF = 2.9 Hz), 143.0, 149.6, 163.0 (1JCF = 245.6 Hz), 163.9 (1JCF = 247.5 Hz); 19F-NMR (282 MHz, DMSO-d6) −109.4 (1F, ddd, J = 4.7, 8.0 and 19.7 Hz), −133.74 (1F, ddd, J = 4.7, 8.0 and 19.8 Hz); HRMS (ES): MH+, found: 386.1214. C22H14N5F2+ requires 386.1217. Anal. calcd. for C16H13N5F2: C, 68.57; H, 3.40; N, 18.17. Found: C, 68.56; H, 3.37; N, 18.16.
(E)-5-(4-Chlorostyryl)-9-(4-fluorophenyl)tetrazolo[1,5-c]quinazoline (4c). Solid (0.11 g, 50%), Rf 0.56, m.p. 238–240 °C; νmax (ATR) 473, 510, 766, 829, 981, 1074, 1296, 1387, 1469, 1501, 1582, 1633, 3069 cm−1; δH (500 MHz, DMSO-d6) 7.03 (1H, d, J = 16.5 Hz, Hb), 7.32 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.52 (2H, d, J = 9.0 Hz, 3″,5″-H), 7.68 (2H, d, J = 9.0 Hz, 2′,6′-H), 7.76 (1H, d, J = 8.0 Hz, 7-H), 8.82 (2H, t, J = 9.0 Hz, 2″,6″-H), 7.51 (1H, d, Jtrans = 16.5 Hz, Ha), 8.12 (1H, dd, J = 2.0 Hz and 8.0 Hz, 8-H), 8.29 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 116.1 (d, 2JCF = 21.8 Hz), 121.1, 121.8, 124.2, 127.7, 128.1, 128.9, 129.4 (3JCF = 8.5 Hz), 129.6, 130.4, 132.6, 133.9 (d, 4JCF = 2.9 Hz), 134.0, 135.4, 135.8, 139.1, 151.7, 163.3 (1JCF = 243.6 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.7 (1F, ddd, J = 6.6, 9.9 and 19.7 Hz); HRMS (ES): MH+, found: 402.0897. C24H14N5F35Cl+ requires 402.0922. Anal. calcd. for C16H13N5F2: C, 65.76; H, 3.26; N, 17.43. Found: C, 65.73; H, 3.25; N, 17.42.
9-((E)-4-Fluorostyryl)-5-((E)-styryl)tetrazolo[1,5-c]quinazoline (4d). Solid (0.15 g, 47%), Rf 0.51, m.p. 213–215 °C; νmax (ATR) 520, 591, 691, 754, 832, 980, 1235, 1501, 1634, 3025 cm−1; δH (500 MHz, DMSO-d6) 7.24 (2H, t, J = 8.5 Hz, 3″,5″-H), 7.37 (1H, t, J = 5.0 Hz, 4′), 7.38 (1H, d, Jtrans = 16.0 Hz, Hb), 7.50 (2H, d, J = 7.0 Hz, 3′,5′-H), 7.52 (1H, d, Jtrans = 16.0 Hz, Hb), 7.58 (1H, d, Jtrans = 16.0 Hz, Ha), 7.73 (2H, t, J 7.0 = Hz, 2′,6′-H), 7.89 (2H, t, J = 7.0 Hz, 2″,6″-H), 8.10 (1H, d, J = 8.5 Hz, 7-H), 8.27 (1H, dd, J = 1.5 Hz and 8.5 Hz, 8-H), 8.35 (1H, d, Jtrans = 16.0 Hz, Ha), 8.69 (1H, d, J = 1.5 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 114.9, 116.5 (d, 2JCF = 20.8 Hz), 129.1, 129.3, 129.4, 129.5, 129.6, 129.9 (2 × C), 130.0 (d, 2JCF = 20.9 Hz), 130.6, 132.0, 132.9 (d, 4JCF = 2.8 Hz), 134.9, 137.2, 140.6, 141.6, 146.8, 148.7, 163.0 (d, 1JCF = 245.6 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.71 (1F, ddd, J = 6.6, 9.9, 19.7 Hz); HRMS (ES): MH+, found: 394.1468. C24H17N5F+ requires: 394.1455. Anal. calcd. for C16H16N5F2: C, 73.27; H, 4.10; N, 17.80. Found: C, 73.24; H, 4.07; N, 17.78.
5,9-Bis((E)-4-fluorostyryl)tetrazolo[1,5-c]quinazoline (4e). Solid (0.25 g, 58%), Rf 0.80, m.p. 241–243 °C; νmax (ATR) 508, 819, 971, 1159, 1233, 1484, 1509, 1635, 2853, 2924, 3045 cm−1; δH (300 MHz, DMSO-d6) 7.22 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.30 (2H, t, J = 9.0 Hz, 3″,5″-H), 7.45 (1H, d Jtrans = 16.0 Hz, Hb), 7.54 (1H, d, Jtrans = 16.0 Hz, Hb), 7.69 (2H, t, J = 7.5 Hz, 2′,6′-H), 7.80 (1H, d, Jtrans = 16.0 Hz, Ha), 7.96 (2H, t, J = 7.5 Hz, 2″,6″-H), 8.03 (1H, d, J = 8.5 Hz, 7-H), 8.21 (1H, dd, J = 1.5 and 8.5 Hz, 8-H), 8.28 (1H, d, Jtrans = 16.0 Hz, Ha), 8.58 (1H, d, J = 1.5 Hz, 10-H); 13C-NMR (75 MHz, DMSO-d6) 115.5, 116.1 (2JCF = 20.9 Hz), 116.6 (2JCF = 21.9 Hz), 122.0, 127.1, 128.8, 129.0 (d, 3JCF = 8.6 Hz), 130.9, 131.3, 131.5 (d, 3JCF = 8.5 Hz), 131.6, 131.8 (d, 4JCF = 2.8 Hz), 133.7 (d, 4JCF = 3.0 Hz), 138.4, 141.0, 142.5, 142.6, 149.5, 162.0 (d, 1JCF = 248.0 Hz), 163.8 (d, 1JCF = 247.4 Hz); 19F-NMR (282 MHz, DMSO-d6) −109.5 (1F, ddd, J = 5.2, 8.5 and 18.3 Hz), −110.2 (1F, ddd, J = 5.2, 9.8 and 15.0 Hz); HRMS (ES): MH+, found: 412.1366. C24H16N535F2+ requires 412.1374. Anal. calcd. for C16H15N5F2: C, 70.07; H, 3.67; N, 17.02. Found: C, 70.05; H, 3.66; N, 17.00.
5-((E)-4-Chlorostyryl)-9-((E)-4-fluorostyryl)tetrazolo[1,5-c]quinazoline (4f). Solid (0.12 g, 55%), Rf 0.51, m.p. 238–241 °C; νmax (ATR) 515, 592, 789, 814, 840, 970, 1014, 1074, 1096, 1234, 1499, 1589, 1636 cm−1; δH (500 MHz, DMSO-d6) 7.22 (2H, t, J = 7.5 Hz, 3″,5″-H), 7.45 (1H, d, Jtrans = 16.0 Hz, Hb), 7.51 (2H, d, J = 7.5 Hz, 3′,5′-H), 7.54 (1H, d, Jtrans = 16.0 Hz, Hb′), 7.70 (2H, t, J = 7.5 Hz, 2′,4′-H), 7.86 (1H, d, Jtrans = 16.0 Hz, Ha), 7.90 (2H, d, J = 7.5 Hz, 2″,6-H), 8.04 (1H, d, J = 8.5 Hz, 7-H), 8.23 (1H, dd, J = 1.5 and 8.5 Hz, 8-H), 8.28 (1H, d, Jtrans = 16.0 Hz, Ha′), 8.59 (1H, d, J 1.5 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 116.3 (d, 2JCF = 21.7 Hz), 121.4, 123.4, 127.7, 129.2 (d, 3JCF = 8.0 Hz), 129.7, 129.5, 133.1, 135.8 (d, 4JCF = 3.0 Hz), 136.9, 130.4, 132.6, 133.9 (d, 4JCF = 2.9 Hz), 134.0, 135.4, 135.8, 139.1, 148.6, 154.9, 163.1 (d, 1JCF = 242.5 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.71 (1F, ddd, J = 6.6, 10.0, 19.7 Hz); HRMS (ES): MH+, found: 428.1073. C24H16N535ClF+ requires: 428.1078. Anal. calcd. for C16H15N5FCl, 67.37; H, 3.53; N, 16.37. Found: C, 67.35; H, 3.49; N, 16.34.
3.4. Typical Procedure for the One-Pot SNAr-Heteroannulation and Suzuki-Miyaura Cross-Coupling of 2a–c to Afford 4a–f
A mixture of
2a (0.20 g, 0.58 mmol) and sodium azide (0.04 g, 0.62 mmol) was stirred at 45 °C for 4 h. 4-Fluorophenylboronic acid (0.12 g, 0.86 mmol), PdCl
2(P(Cy)
3)
2 (0.04 g, 0.058 mmol), K
2CO
3 (1.00 g, 0.70 mmol) and water (15 mL) were then added to the mixture under nitrogen atmosphere. The mixture was heated at 100 °C for 3 h and then poured into ice-cold water. The product was extracted into chloroform and the combined organic phases were washed with brine, dried over MgSO
4 and the salt was filtered off. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel. Compounds
4a–
f were prepared in this fashion; see
Table 1 under
Scheme 2 for the corresponding yields.
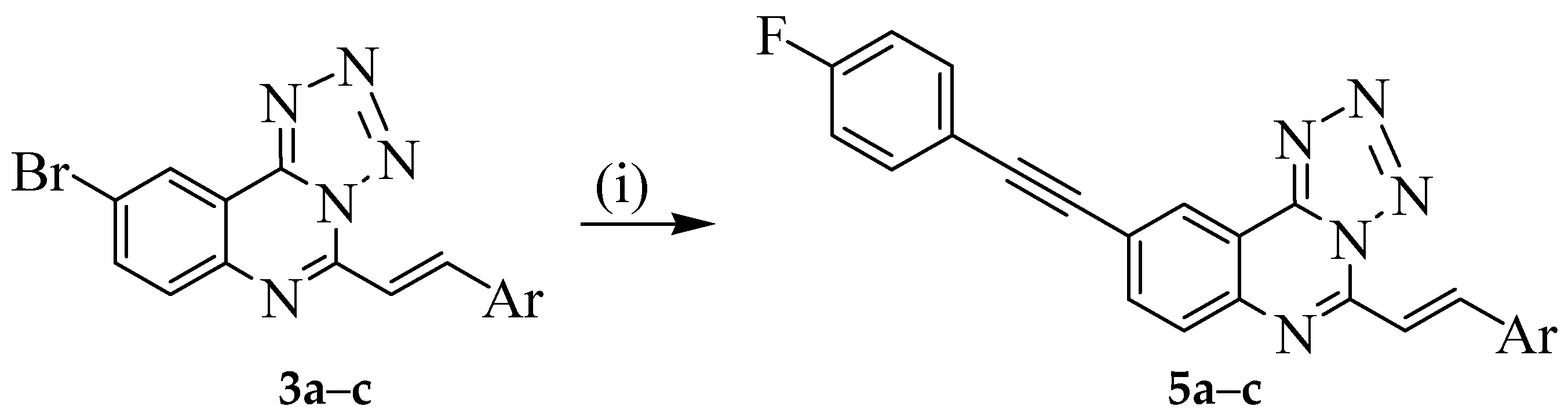
3.5. Typical Procedure for the Sonogashira Cross-Coupling of 3a–c; Synthesis of 5a–c
A mixture of 3a (0.30 g, 0.86 mmol), 4-fluorophenylacetylene (0.16 g, 1.30 mmol), PdCl2(P(Cy)3)2 (0.06 g, 0.08 mmol), CuI (0.016 g, 0.08 mmol) and Cs2CO3 (0.40 g, 0.13 mmol) in DMF (15 mL) was heated at 100 °C for 2 h under nitrogen atmosphere. The mixture was then poured into ice-cold water. The resultant precipitate was filtered and purified by column chromatography on silica gel using 9:1 toluene–ethyl acetate (v/v) as an eluent. Compounds 5a–c were prepared in this fashion.
(E)-9-((4-Fluorophenyl)ethynyl)-5-styryltetrazolo[1,5-c]quinazoline (5a). Solid (0.17 g, 50%), Rf 0.70, m.p. 175–177 °C; νmax (ATR) 467, 686, 750, 829, 975, 1229, 1506, 1588, 1630, 2202, 2923 cm−1; δH (500 MHz, DMSO-d6) 7.30 (2H, t, J = 9.0 Hz, 3″,5″-H), 7.47–7.47 (3H, m, Ph), 7.72 (2H, t, J = 9.0 Hz, 2″,6″-H), 7.87 (1H, d, Jtrans = 16.0 Hz, Hb), 7.85–7.91 (2H, m, Ph), 8.08 (1H, d, J = 9.0 Hz, 7-H), 8.16 (1H, dd, J = 1.8 Hz and 9.0 Hz, 8-H), 8.04 (1H, d, Jtrans = 16.5 Hz, Ha), 8.65 (1H, d, J = 1.8 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 88.4, 91.9, 115.5, 115.6 (d, 2JCF = 21.9 Hz), 115.7, 118.6 (d, 4JCF = 2.9 Hz), 121.7, 129.0, 129.4, 129.7 (2 × C), 131.3, 134.6 (d, 3JCF = 8.5 Hz), 135.0, 136.4, 142.9, 143.1, 143.7, 149.0, 163.19 (d, 1JCF = 245.7 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.16 (1F, d, J = 4.7 Hz); HRMS (ES): MH+, found: 392.1304. C24H15N5F+ requires: 392.1311. Anal. calcd. for C16H14N5F: C, 73.65; H, 3.61; N, 17.89. Found: C, 73.59; H, 3.60; N, 17.64.
(E)-9-((4-Fluorophenyl)ethynyl)-5-(4-fluorostyryl)tetrazolo[1,5-c]quinazoline (5b). Solid (0.12 g, 48%), Rf 0.54, m.p. 204–206 °C; νmax (ATR) 508, 812, 834, 1234, 1506, 1588, 1635, 2361, 3023 cm−1; δH (300 MHz, DMSO-d6) 7.33 (2H, t, J = 9.0 Hz, 3′,5′-H), 7.33 (2H, t, J = 9.0 Hz, 3″,5″-H), 7.73 (2H, t, J = 9.0 Hz, 2′,6′-H), 7.91 (1H, d, Jtrans = 16.0 Hz, Hb), 8.04 (2H, t, J = 9.0 Hz, 2″,6″-H), 8.12 (1H, dd, J = 1.2 and 8.4 Hz, 8-H), 8.12 (1H, d, J = 8.4 Hz, 7-H), 8.39 (1H, d, Jtrans = 16.0 Hz, Ha), 8.67 (1H, d, J = 1.2 Hz, 10-H); 13C-NMR (75 MHz, DMSO-d6) 88.8, 90.4, 116.3 (d, 2JCF = 21.8 Hz), 116.5 (d, 2JCF = 21.8 Hz), 118.8 (d, 4JCF = 3.0 Hz), 120.9, 126.9, 128.4, 129.1, 129.3, 129.6 (d, 4JCF = 2.8 Hz), 129.9, 134.1, 134.4 (d, 3JCF = 8.5 Hz), 134.5 (d, 3JCF = 8.5 Hz), 136.6, 137.4, 151.2, 161.4 d, 1JCF = 245.7 Hz), 163.1 (d, 1JCF = 244.7 Hz); 19F-NMR (282 MHz, DMSO-d6) −109.2 (1F, ddd, J = 6.6, 9.9 and 20.2 Hz), −109.5 (1F, ddd, J = 3.3, 8.5, 16.5 Hz); HRMS (ES): MH+, found: 410.1216. C24H14N5F2+ requires: 410.1217. Anal. calcd. for C16H13N5F2: C, 70.41; H, 3.20; N, 17.11. Found: C, 70.39; H, 2.99; N, 17.01.
(E)-5-(4-Chlorostyryl)-9-((4-fluorophenyl)ethynyl)tetrazolo[1,5-c]quinazoline (5c). Solid (0.16 g, 52%), Rf 0.61, m.p. 235–237 °C; νmax (ATR) 520, 820, 1091, 1232, 1495, 1587, 1633, 2216, 3059 cm−1; δH (500 MHz, DMSO-d6) 7.35 (2H, t, J 8.7 Hz, 3″,5″-H), 7.52 (2H, d, J = 8.1 Hz, 3′,5′-H), 7.89 (1H, d, Jtrans = 16.0 Hz, Hb), 7.92 (2H, d, J = 8.1 Hz, 2′,6′-H), 7.93 (2H, t, J 8.7 Hz, 2″,6″-H), 8.13 (1H, d, J = 8.1 Hz, 7-H), 8.30 (1H, d, Jtrans = 16.0 Hz, Ha), 8.28 (1H, dd, J = 1.2 Hz and 9.0 Hz, 8-H), 8.67 (1H, d, J = 1.2 Hz, 10-H); 13C-NMR (125 MHz, DMSO-d6) 88.4, 90.4, 115.5, 115.6 (d, 2JCF = 21.9 Hz), 115.7, 118.6 (d, 4JCF = 2.9 Hz), 123.1, 127.1, 129.1, 129.2, 129.7, 131.3, 134.6 (d, 3JCF = 8.5 Hz), 135.0, 136.4, 142.9, 143.1, 143.7, 149.0, 162.9 (d, 1JCF = 247.5 Hz); 19F-NMR (282 MHz, DMSO-d6) −113.70 (1F, dd, J = 4.7 and 14.6 Hz); HRMS (ES): MH+, found: 426.0916. C24H14N535ClF+ requires: 426.0922. Anal. calcd. for C16H13N5FCl: C, 67.69; H, 3.08; N, 16.45. Found: C, 67.70; H, 3.06; N, 16.41.
3.6. Typical Procedure for the One-Pot SNAr-Heteroannulation and Sonogashira Cross-Coupling of 2a–c to Afford 5a–c
A mixture of
2a (0.40 g, 1.16 mmol) and sodium azide (0.08 g, 1.24 mmol) in DMF ( 20 mL) was stirred at 45 °C for 4 h. 4-Fluorophenylacetylene (0.21 g, 1.71 mmol), PdCl
2(P(Cy)
3)
2 (0.04 g, 0.06 mmol), CuI (0.01 g, 0.06 mmol), Cs
2CO
3 (0.45 g, 1.37 mmol) and water (5 mL) were added to the mixture under nitrogen atmosphere. The mixture was heated at 90 °C for 4 h and then poured into ice-cold water. The product was extracted into chloroform and the combined organic phases were washed with brine, dried over MgSO
4 and the salt was filtered off. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel. Compounds
5a–
c were prepared in this fashion; see
Table 2 under
Scheme 3 for the corresponding yields.
3.7. Cytotoxicity Screening Protocol
The human tumour cell lines, HeLa and MCF7, were grown in RPMI and DMEM respectively and supplemented with 10% foetal bovine serum and 2 mM l-glutamine. For a screening experiment, cells were inoculated into 96 well microtiter plates at a density of 6000 cells/well using a volume of 100 μL in each well. After cell inoculation, the microtiter plates were incubated at 37 °C, 5% CO2, and 100% relative humidity for approximately 24 h prior to addition of test compounds. At the time of test compound addition, the samples were diluted to double the desired final maximum test concentration with complete medium and additional dilutions were made to provide five drug concentrations. Aliquots of 100 µL of these different drug dilutions were added to the appropriate microtiter wells already containing 100 µL of medium, resulting in the required final drug concentrations. Following test sample addition, the plates were incubated for a further 48 h at 37 °C, 5% CO2, in a humidified incubator. At the end of this incubation period the medium was removed and replaced with fresh culture medium containing MTT at a final concentration of 0.5 mg/mL. The 96-well plates were returned to the incubator and incubated for an additional 3 h following which the medium was removed, the MTT crystals solubilized in DMSO and absorbance measured at 560 nm using a BioTek PowerWave XS Spectrophotometer (BioTech Instrument, Winooski, VT, USA).
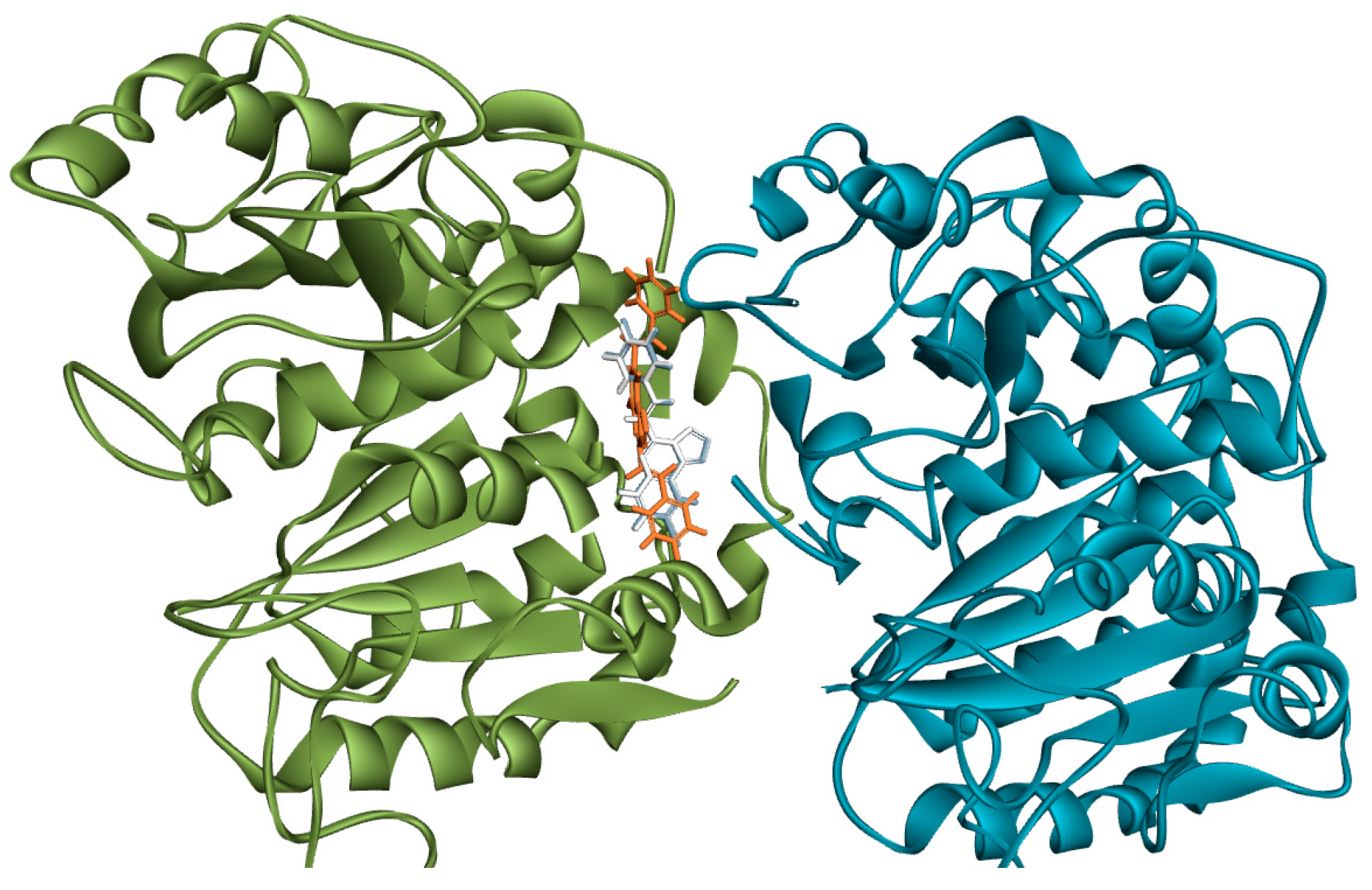
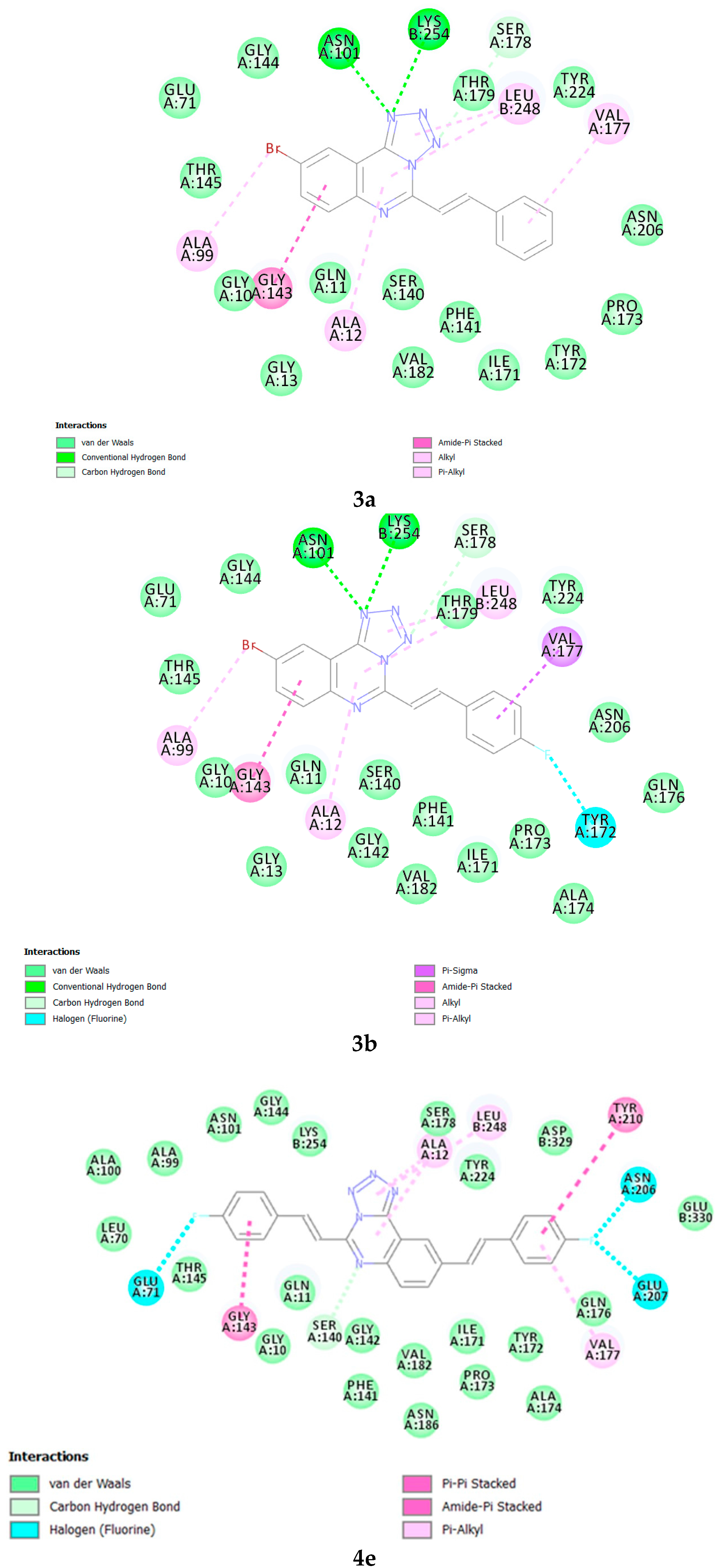
3.8. Methodology for Docking Studies
Molecular docking of compounds
3a,
3b and
4e to the 3D structure of a tubulin heterodimer (PDB code:1TUB [
15] was carried out using the CDOCKER protocol [
16] in Discovery Studio 2017. Prior to performing the docking, compounds were drawn using Discovery Studio and prepared using the ‘Prepare Ligand’ protocol. The protein structure was downloaded from the Protein Data Bank, prepared using the ‘Prepare Protein’ protocol in Discovery Studio which included removing any existing ligands bound to the model and binding sites defined from receptor cavities. The receptor cavity in this tubulin structure is present between the two monomers of the tubulin dimer structure. Docking was performed using default settings and the best conformation of the ligand selected and evaluated.