New Adducts of Iriflophene and Flavonoids Isolated from Sedum aizoon L. with Potential Antitumor Activity
Abstract
:1. Introduction
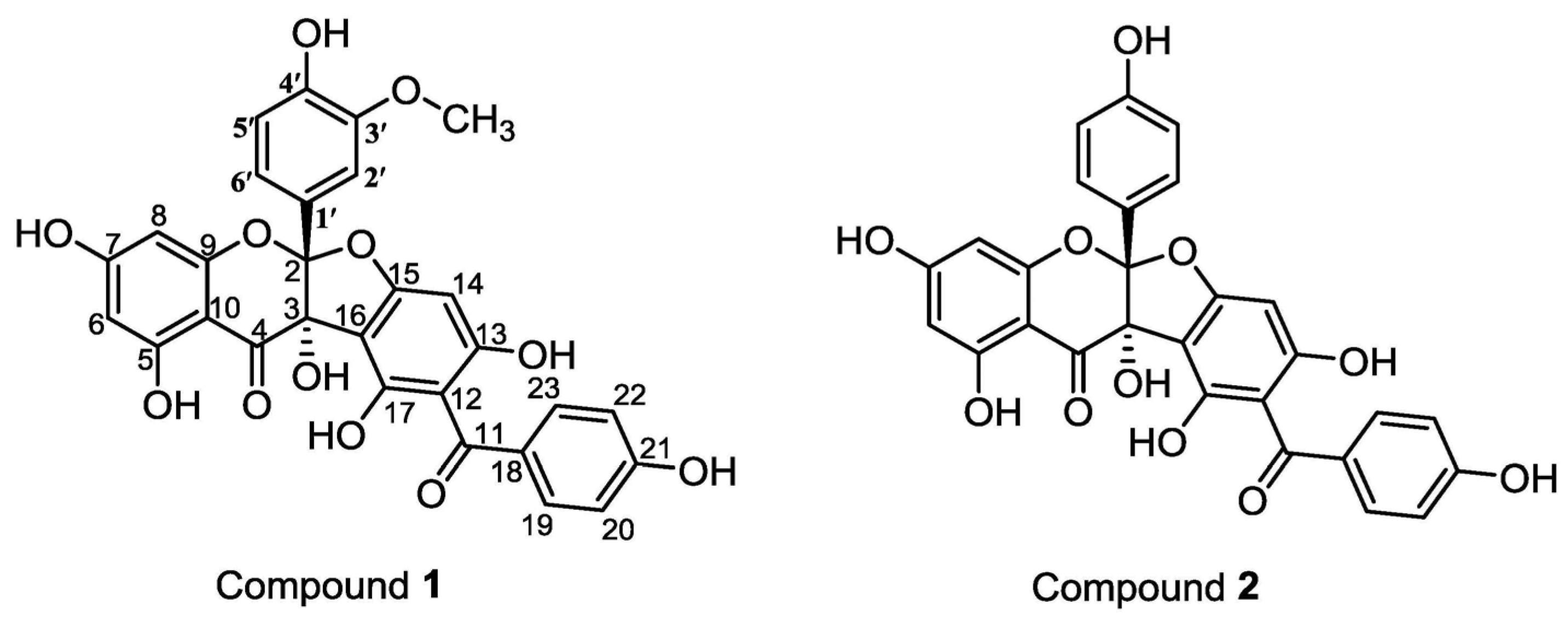
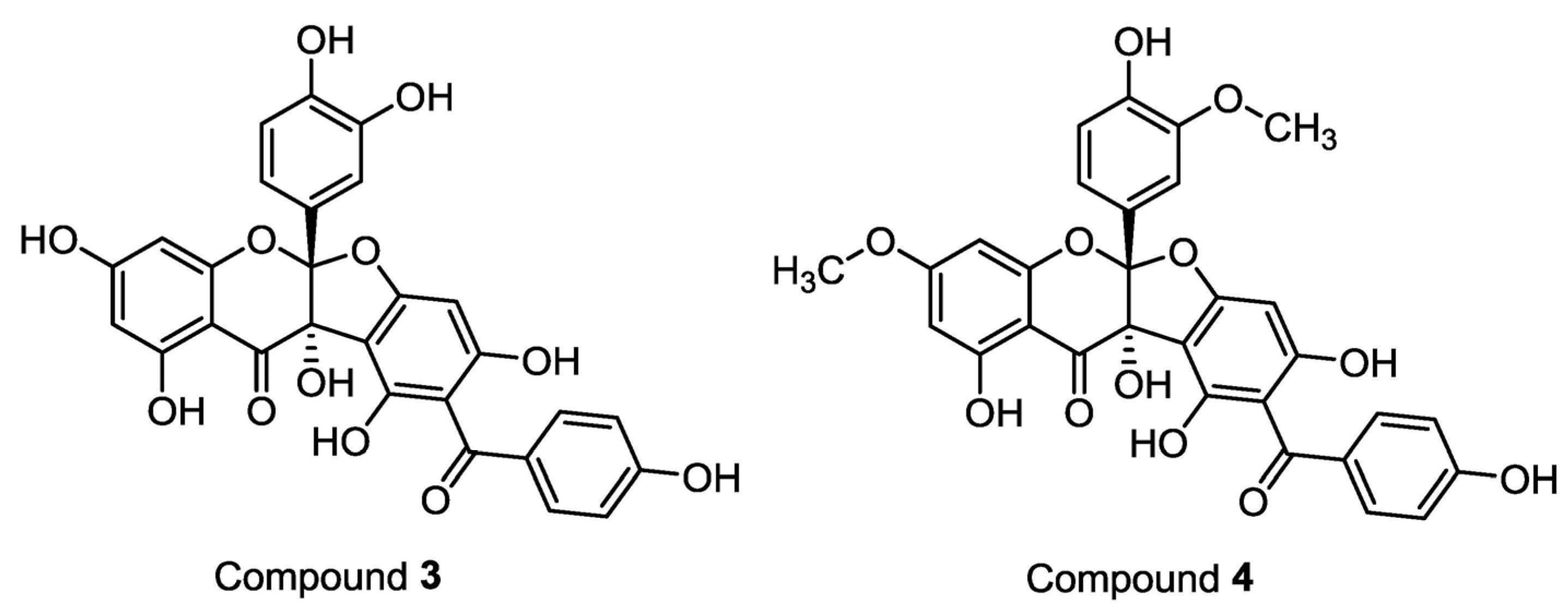
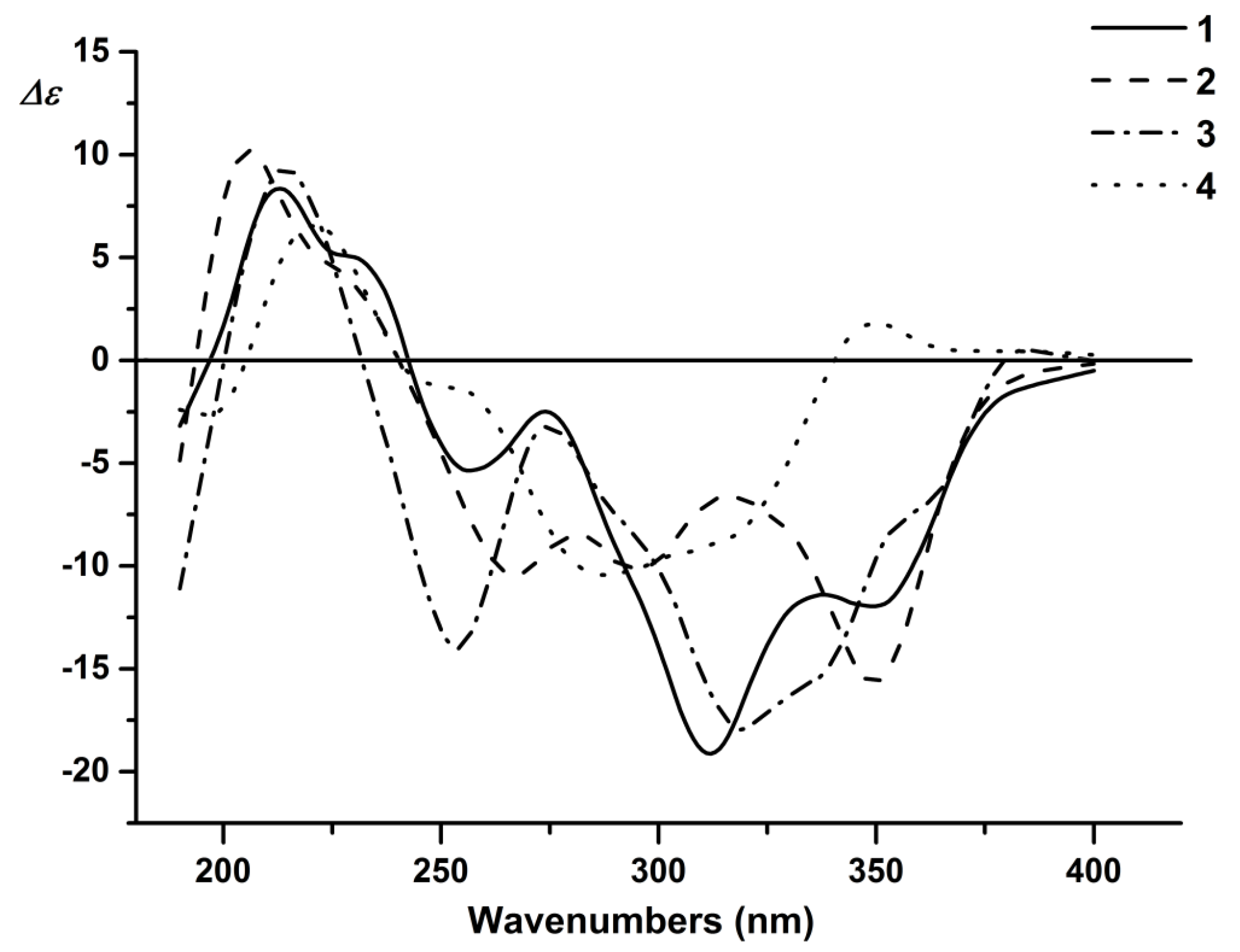
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.Y.; Gao, H. Tusanqi and hepatic sinusoidal obstruction syndrome. J. Digest. Dis. 2014, 15, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chen, H.Z.; Zhu, J.S.; Ruan, B.; Zhang, Z.Q.; Lin, X.; Gan, M.F. Computed tomography findings of hepatic veno-occlusive disease caused by Sedum aizoon with histopathological correlation. Braz. J. Med. Biol. Res. 2015, 48, 1145. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wei, X.; Yang, S.; Yao, L.; Gong, G. Microwave-assisted Extraction and Antioxidant Activity of Flavonoids from Sedum aizoon Leaves. Food Sci. Technol. Res. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Kim, J.H.; Hart, H.T.; Stevens, J.F. Alkaloids of some Asian Sedum species. Phytochemistry 1996, 41, 1319–1324. [Google Scholar] [CrossRef]
- Niu, X.F.; Liu, X.; Pan, L.; Qi, L. Oleanene triterpenes from Sedum lineare Thunb. Fitoterapia 2011, 82, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Luo, Q.Y.; Wu, L.Q. Two new prenylated isoflavones from Sedum aizoon L. Fitoterapia 2011, 82, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Luo, Q.Y.; Wu, L.Q. Two new flavonol glycosides from Sedum aizoon L. Heterocycles 2011, 83, 135. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Huang, A.; Che, L.; Guo, S.; Che, J. Chemical constituents from Sedum aizoon and their hemostatic activity. Pharm. Biol. 2014, 52, 1429–1434. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Z.; Lei, T.; Lv, C.; Wang, J.; Lu, J. New flavonoid glycosides from Sedum aizoon L. Fitoterapia 2015, 101, 125. [Google Scholar] [CrossRef] [PubMed]
- Canuto, K.M.; Leal, L.K.A.M.; Lopes, A.A.; Coleman, C.M.; Ferreira, D.; Silveira, E.R. Amburanins A and B from Amburana cearensis: Daphnodorin-type biflavonoids that modulate human neutrophil degranulation. J. Braz. Chem. Soc. 2014, 25, 639–647. [Google Scholar] [CrossRef]
- Baba, K.; Yoshikawa, M.; Taniguchi, M.; Kozawa, M. Biflavonoids from Daphne odora. Phytochemistry 1995, 38, 1021–1026. [Google Scholar] [CrossRef]
- Taniguchi, M.; Fujiwara, A.; Baba, K. Three flavonoids from Daphne odora. Phytochemistry 1997, 45, 183–188. [Google Scholar] [CrossRef]
- Taniguchi, M.; Fujiwara, A.; Baba, K.; Wang, N.H. Two biflavonoids from Daphne acutiloba. Phytochemistry 1998, 49, 863–867. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, S.W.; Liu, S.S.; Cong, H.J.; Xuan, L.J. Daphnodorin dimers from Edgeworthia chrysantha with α-glucosidase inhibitory activity. Phytochem. Lett. 2010, 3, 242–247. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Li, W.L.; Wu, L.Q. Acylated flavonol glycosides from Sedum aizoon. Chem. Nat. Compd. 2012, 48, 23–25. [Google Scholar] [CrossRef]
- Yuan, H.; Bi, K.; Chang, W.; Yue, R.; Li, B.; Ye, J.; Sun, Q.; Jin, H.; Shan, L.; Zhang, W. Total synthesis of Daphnodorin A. Tetrahedron 2014, 70, 9084–9092. [Google Scholar] [CrossRef]
- Jia, B.X.; Zeng, X.L.; Ren, F.X.; Jia, L.; Chen, X.Q.; Yang, J.; Liu, H.M.; Wang, Q. Baeckeins F-I, four novel C-methylated biflavonoids from the roots of Baeckea frutescens and their anti-inflammatory activities. Food Chem. 2014, 155, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, D.; Chen, W.; Xi, Z.; Chen, C.; Huang, G.; Sun, L. Two new phenolic glycosides from Gnaphalium affine D. Don and their anti-complementary activity. Molecules 2013, 18, 7751–7760. [Google Scholar] [CrossRef] [PubMed]
- Znati, M.; Ben, J.H.; Cazaux, S.; Souchard, J.P.; Harzallah, S.F.; Bouajila, J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.H.; Wojcińska, M.; Drostkarbowska, K.; Matławska, I.; Williams, J.; Mabry, T.J. Kaempferol triosides from Silphium perfoliatum. Phytochemistry 2002, 60, 835. [Google Scholar] [CrossRef]
- Lallemand, J.Y.; Duteil, M. C13 NMR Spectra of Quercetin and Rutin. Magn. Reson. Chem. 1977, 9, 179–180. [Google Scholar] [CrossRef]
- Itokawa, H.; Oshida, Y.; Ikuta, A.; Inatomi, H.; Ikegami, S. Flavonol glycosides from the flowers of Cucurbita pepo. Phytochemistry 1981, 20, 2421–2422. [Google Scholar] [CrossRef]
- Taniguchi, M.; Baba, K. Three biflavonoids from Daphne odora. Phytochemistry 1996, 42, 1447–1453. [Google Scholar] [CrossRef]
- Li, Q.; Gao, W.; Cao, J.; Bi, X.; Chen, G.; Zhang, X.; Xia, X.; Zhao, Y. New cytotoxic compounds from flowers of Lawsonia inermis L. Fitoterapia 2014, 94, 148–154. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Compound | IC50 (μmol L−1) | ||
|---|---|---|---|
| BXPC-3 | MCF-7 | A549 | |
| 1 | >100 | >100 | >100 |
| 2 | >100 | >100 | >100 |
| 3 | 24.84 | 35.89 | 37.20 |
| 4 | 31.22 | 33.90 | 26.11 |
| 5-FU | 15.81 | 17.36 | 2.96 |
| Position | 1 a | 2 b | ||
|---|---|---|---|---|
| δC | δH | δC Type COSY | δH | |
| 4 | 191.25 | 191.64 | ||
| 11 | 191.02 | 191.64 | ||
| 7 | 168.50 | 168.26 | ||
| 5 | 163.29 | 163.75 | ||
| 21 | 162.05 | 162.57 | ||
| 15 | 160.69 | 161.15 | ||
| 9 | 160.45 | 160.75 | ||
| 17 | 159.62 | 160.07 | ||
| 13 | 157.7 | 158.10 | ||
| 4′ | 147.63 | 158.77 | ||
| 3′ | 146.72 | 114.93 | ||
| 19, 23 | 132.06 | 7.72 (d, J = 8.7 Hz, 2H) | 132.54 | 7.70 (d, J = 8.4 Hz, 2H) |
| 18 | 129.70 | 130.00 | ||
| 1′ | 124.81 | 124.67 | ||
| 6′ | 119.05 | 6.61 (dd, J = 8.3, 1.9 Hz,1H) | 128.57 | 7.06 (d, J = 8.4 Hz, 2H) |
| 2 | 117.01 | 117.76 | ||
| 20, 22 | 114.83 | 6.85 (d, J = 8.6 Hz, 2H) | 115.36 | 6.85 (d, J = 8.4 Hz, 2H) |
| 5′ | 114.61 | 6.66 (d, J = 8.3 Hz, 1H) | 114.93 | 6.67 (d, J = 8.4 Hz, 2H) |
| 2′ | 111.62 | 6.76 (d, J = 1.9 Hz, 1H) | 128.57 | |
| 16 | 106.31 | 106.84 | ||
| 12 | 103.23 | 104.00 | ||
| 10 | 98.08 | 98.76 | ||
| 14 | 97.66 | 6.03 (s, 1H) | 97.96 | 6.04 (s, 1H) |
| 6 | 96.85 | 5.86 (s, 1H) | 97.10 | 5.90 (d, J = 2.0 Hz, 1H) |
| 8 | 95.23 | 5.77 (s, 1H) | 95.28 | 5.80 (d, J = 2.0 Hz, 1H) |
| 3 | 79.89 | 80.32 | ||
| 3′-OCH3 | 55.61 | 3.60 (s, 3H) | ||
| Position | 3 a | 4 b | ||
|---|---|---|---|---|
| δC | δH | δC Type COSY | δH | |
| 4 | 192.53 | 192.89 | ||
| 11 | 191.55 | 191.64 | ||
| 7 | 167.30 | 167.88 | ||
| 5 | 163.66 | 163.25 | ||
| 21 | 162.64 | 162.52 | ||
| 15 | 161.36 | 161.32 | ||
| 9 | 160.46 | 161.10 | ||
| 17 | 160.02 | 160.29 | ||
| 13 | 157.64 | 157.50 | ||
| 3′ | 146.91 | 148.20 | ||
| 4′ | 144.87 | 147.26 | ||
| 19, 23 | 132.58 | 7.71 (d, J =8.7 Hz, 2H) | 132.56 | 7.73 (d, J = 8.6 Hz, 2H) |
| 18 | 129.92 | 130.18 | ||
| 1′ | 124.97 | 124.85 | ||
| 6′ | 118.40 | 6.53 (dd, J = 8.3, 2.2 Hz,1H) | 119.36 | 6.60 (dd, J = 8.6, 2.0 Hz, 1H) |
| 2 | 117.91 | 117.94 | ||
| 20, 22 | 115.43 | 6.87 (d, J = 8.7 Hz,2H) | 115.33 | 6.86 (d, J = 8.6 Hz, 2H) |
| 5′ | 115.13 | 6.63 (d, J = 8.3 Hz, 1H) | 115.15 | 6.67 (d, J = 8.3 Hz, 1H) |
| 2′ | 114.80 | 6.70 (d, J = 8.3 Hz, 1H) | 111.91 | 6.75 (d, J = 2.0 Hz, 1H) |
| 16 | 106.94 | 106.80 | ||
| 12 | 104.26 | 103.79 | ||
| 10 | 98.98 | 99.92 | ||
| 14 | 97.87 | 6.06 (s, 1H) | 98.17 | 6.04 (s, 1H) |
| 6 | 96.79 | 5.95 (d, J = 2.0 Hz, 1H) | 95.81 | 6.13 (d, J = 1.7 Hz, 1H) |
| 8 | 94.97 | 5.85 (d, J = 2.0 Hz, 1H) | 93.75 | 6.07 (d, J = 1.7 Hz, 1H) |
| 7-OCH3 | 94.97 | 56.07 | 3.77 (s, 3H) | |
| 3-OCH3 | 94.97 | 55.37 | 3.61 (s, 3H) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Qi, Z.; Hao, Y.; Lv, C.; Jia, L.; Wang, J.; Lu, J. New Adducts of Iriflophene and Flavonoids Isolated from Sedum aizoon L. with Potential Antitumor Activity. Molecules 2017, 22, 1859. https://doi.org/10.3390/molecules22111859
Li M, Qi Z, Hao Y, Lv C, Jia L, Wang J, Lu J. New Adducts of Iriflophene and Flavonoids Isolated from Sedum aizoon L. with Potential Antitumor Activity. Molecules. 2017; 22(11):1859. https://doi.org/10.3390/molecules22111859
Chicago/Turabian StyleLi, Mingxiao, Zheyuan Qi, Yimeng Hao, Chongning Lv, Lingyun Jia, Jing Wang, and Jincai Lu. 2017. "New Adducts of Iriflophene and Flavonoids Isolated from Sedum aizoon L. with Potential Antitumor Activity" Molecules 22, no. 11: 1859. https://doi.org/10.3390/molecules22111859





