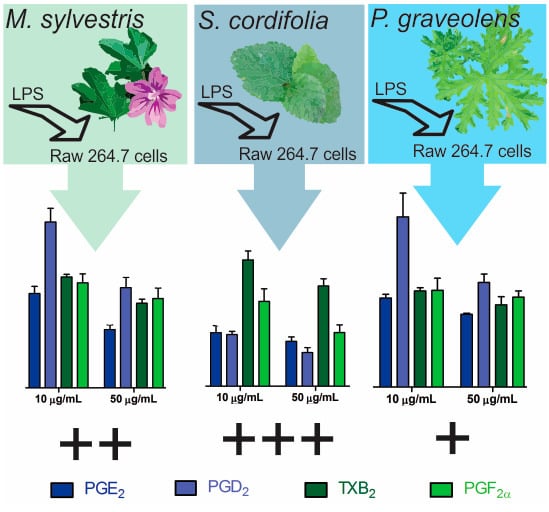
Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production
Abstract
:1. Introduction
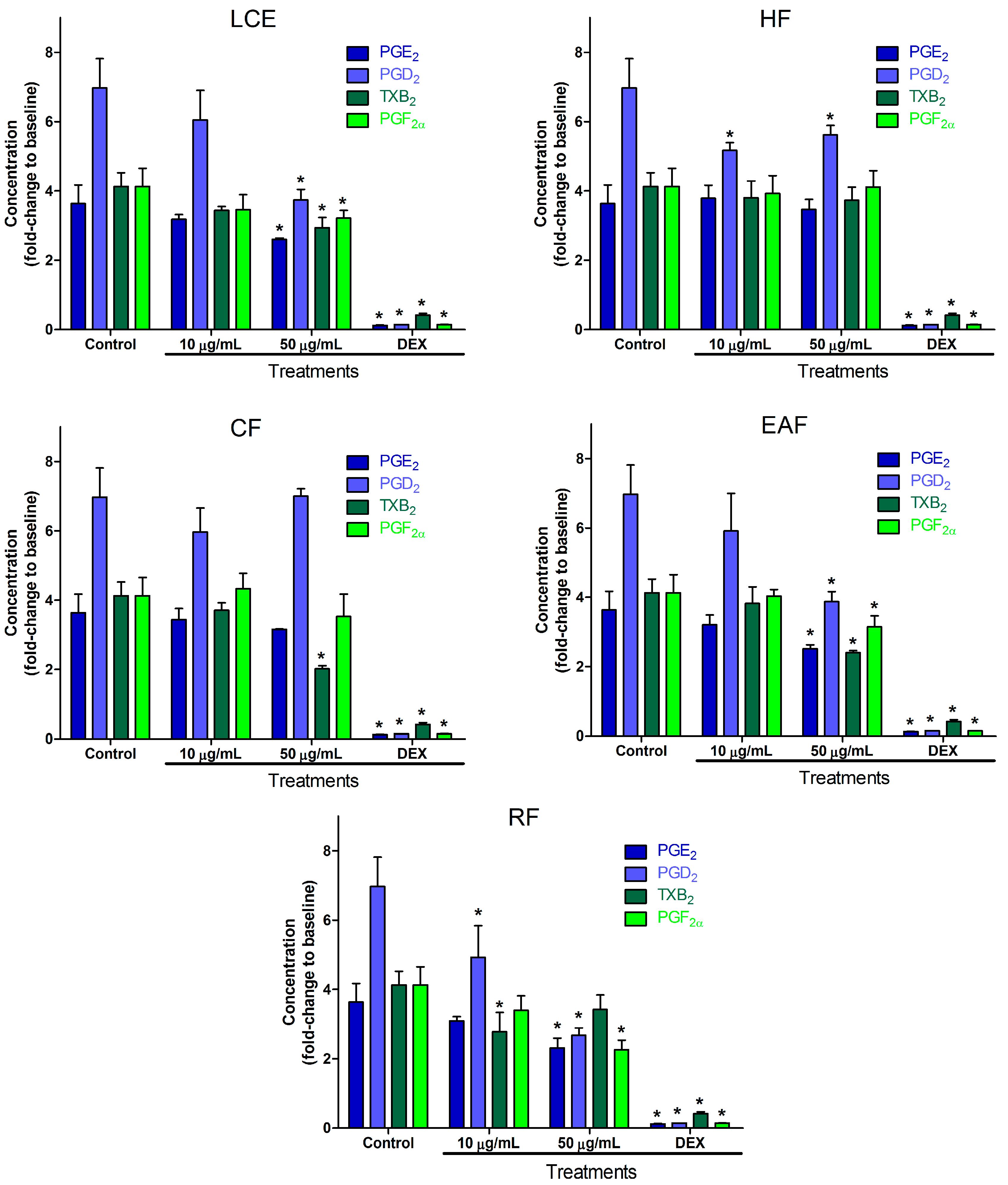
2. Results and Discussion
2.1. RAW 264.7 Cell Viability
2.2. Inflammatory Mediator Release Evaluation
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Extracts and Fractions Obtainment
3.2.1. Plant Material and Ethanolic Extract
3.2.2. Fractions of Leaf Extracts
3.3. Analysis of PGE2, PGD2, PGF2α, and TXB2
3.3.1. Sample Preparation
3.3.2. LC-MS/MS
3.4. Pharmacological Assessment
3.4.1. Cell Culture
3.4.2. Effect of Extracts and Fractions on Cell Viability
3.4.3. Inflammatory Mediator Release Evaluation
3.4.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. The inflammatory mediators. Ann. N. Y. Acad. Sci. 1974, 221, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Oh, D.K. Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 2017, 66, 50–68. [Google Scholar] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Blewett, A.J.; Varma, D.; Gilles, T.; Libonati, J.R.; Jansen, S.A. Development and validation of a high-performance liquid chromatography-electrospray mass spectrometry method for the simultaneous determination of 23 eicosanoids. J. Pharm. Biomed. Anal. 2008, 46, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.F.; Piantavini, M.S.; Ribeiro, R.P.; Amano, E.; Dal Prá, B.V.; Pontarolo, R. Non-targeted electrospray mass spectrometry-based metabolic fingerprinting and pls-da-based classification of brazilian “malvas”. J. Braz. Chem. Soc. 2015, 26, 365–372. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Martins, C.A.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [PubMed]
- Bach, H.; Benso, B.; Franchin, M.; Massarioli, A.P.; Paschoal, J.A.R.; Alencar, S.M.; Franco, G.C.N.; Rosalen, P.L. Anti-inflammatory, anti-osteoclastogenic and antioxidant effects of Malva sylvestris extract and fractions: In vitro and in vivo studies. PLoS ONE 2016, 11, e0162728. [Google Scholar]
- Franzotti, E.M.; Santos, C.V.; Rodrigues, H.M.; Mourao, R.H.; Andrade, M.R.; Antoniolli, A.R. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (malva-branca). J. Ethnopharmacol. 2000, 72, 273–277. [Google Scholar] [CrossRef]
- Dinda, B.; Das, N.; Dinda, S.; Dinda, M.; SilSarma, I. The genus Sida L.—A traditional medicine: Its ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. J. Ethnopharmacol. 2015, 176, 135–176. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, O.J.V.; Perea, E.M.; Mendez, J.J.; Arango, W.M.; Noreña, D.A. Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. (escobilla). Rev. Cuba. Plant. Med. 2013, 18, 16. [Google Scholar]
- Al-Sayed, E.; Martiskainen, O.; Seif el-Din, S.H.; Sabra, A.-N.A.; Hammam, O.A.; El-Lakkany, N.M. Protective effect of Pelargonium graveolens against carbon tetrachloride-induced hepatotoxicity in mice and characterization of its bioactive constituents by hplc–pda–esi–ms/ms analysis. Med. Chem. Res. 2014, 24, 1438–1448. [Google Scholar] [CrossRef]
- Boukhris, M.; Simmonds, M.S.J.; Sayadi, S.; Bouaziz, M. Chemical composition and biological activities of polar extracts and essential oil of rose-scented geranium, Pelargonium graveolens. Phytother. Res. 2013, 27, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Bonsangue, G.; Letizia Gargano, M.; Venturella, G.; La Bella, S. Popular uses of wild plant species for medicinal purposes in the nebrodi regional park (North-Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 157, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Traditional phytotherapy in central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Elsagh, M.; Fartookzadeh, M.R.; Kamalinejad, M.; Anushiravani, M.; Feizi, A.; Behbahani, F.A.; Rafiei, R.; Arjmandpour, A.; Adibi, P. Efficacy of the Malva sylvestris L. Flowers aqueous extract for functional constipation: A placebo-controlled trial. Complement. Ther. Clin. Pract. 2015, 21, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Prudente, A.S.; Sponchiado, G.; Mendes, D.A.G.B.; Soley, B.S.; Cabrini, D.A.; Otuki, M.F. Pre-clinical efficacy assessment of Malva sylvestris on chronic skin inflammation. Biomed. Pharmacother. 2017, 93, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Chiclana, C.F.; Enrique, A.; Consolini, A.E. Topical antiinflammatory activity of Malva sylvestris L. (malvaceae) on carragenin-induced edema in rats. Latin Am. J. Pharm. 2009, 28, 275–278. [Google Scholar]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Loggia, R.D. In vivo anti-inflammatory and in vitro antioxidant activities of mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, N.H.; Daher, C.F. Malva sylvestris water extract: A potential anti-inflammatory and anti-ulcerogenic remedy. Planta Med. 2009, 75. [Google Scholar] [CrossRef]
- Prudente, A.S.; Loddi, A.M.V.; Duarte, M.R.; Santos, A.R.S.; Pochapski, M.T.; Pizzolatti, M.G.; Hayashi, S.S.; Campos, F.R.; Pontarolo, R.; Santos, F.A.; et al. Pre-clinical anti-inflammatory aspects of a cuisine and medicinal millennial herb: Malva sylvestris L. Food Chem. Toxicol. 2013, 58, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Weffort-Santos, A.M.; Gasparetto, J.C.; Trindade, A.C.; Otuki, M.F.; Pontarolo, R. Malva sylvestris L. Extract suppresses desferrioxamine-induced pge(2) and pgd(2) release in differentiated u937 cells: The development and validation of an lc-ms/ms method for prostaglandin quantification. Biomed. Chromatogr. 2014, 28, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Choubev, S.; Singour, P.K.; Rajak, H.; Pawar, R.S. Sida cordifolia (linn)-an overview. J. Appl. Pharm. Sci. 2011, 01, 8. [Google Scholar]
- Singh, A.P. Bala (Sida cordifolia L.)-is it safe herbal drug? Ethnobot. Leafl. 2006, 10, 5. [Google Scholar]
- Kanth, V.R.; Diwan, P.V. Analgesic, antiinflammatory and hypoglycaemic activities of sida cordifolia. Phytother. Res. PTR 1999, 13, 75–77. [Google Scholar] [CrossRef]
- Bonjardim, L.R.; Silva, A.M.; Oliveira, M.G.B.; Guimarães, A.G.; Antoniolli, A.R.; Santana, M.F.; Serafini, M.R.; Santos, R.C.; Araújo, A.A.S.; Estevam, C.S.; et al. Sida cordifolia leaf extract reduces the orofacial nociceptive response in mice. Phytother. Res. 2011, 25, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, R.K.; Rahman, A.M.; Ahmad, M.; Bachar, S.C.; Saha, A.; Guha, S.K. Bioactive alkaloid from Sida cordifolia linn. With analgesc and anti-inflammatory activities. Iran. J. Pharmacol. Ther. 2006, 5, 4. [Google Scholar]
- Sutradhar, R.K.; Rahman, A.M.; Ahmad, M.; Bachar, S.C.; Saha, A.; Roy, T.G. Anti-inflammatory and analgesic alkaloid from Sida cordifolia linn. Pak. J. Pharm. Sci. 2007, 20, 185–188. [Google Scholar] [PubMed]
- Sutradhar, R.K.; Rahman, A.K.M.M.; Ahmad, M.U.; Bachar, S.C. Bioactive flavones of sida cordifolia. Phytochem. Lett. 2008, 1, 179–182. [Google Scholar] [CrossRef]
- Saraswathi, J.; Venkatesh, K.; Baburao, N.; Hilal, M.H.; Rani, A.R. Phytopharmacological importance of pelargonium species. J. Med. Plants Res. 2011, 5, 2587–2598. [Google Scholar]
- Asgarpanah, J.; Ramezanloo, F. An overview on phytopharmacology of Pelargonium graveolens L. Indian J. Tradit. Knowl. 2015, 14, 558–563. [Google Scholar]
- Lavasanijou, M.R.; Sohrabi, H.R.; Karimi, M.; Ashjazade, M.A.; Salajeghe, M.; Farzineejadizadeh, H.; Parsaei, P.; Elmamooz, A. Wound healing effects of quercus brantii and Pelargonium graveolens extracts in male wistar rats. Wounds 2016, 28, 369–375. [Google Scholar] [PubMed]
- Ghanizadeh, B.; Shafaroodi, H.; Asgarpanah, J.; Sharifi, Z.N. The anti-inflammatory effect of Pelargonium graveolens methanolic extract in acetic acid-induced ulcerative colitis in rat model. Clin. Ther. 2015, 37, e51. [Google Scholar] [CrossRef]
- Kreth, J.; Benso, B.; Rosalen, P.L.; Alencar, S.M.; Murata, R.M. Malva sylvestris inhibits inflammatory response in oral human cells. An in vitro infection model. PLoS ONE 2015, 10, e0140331. [Google Scholar]
- Swathy, S.S.; Panicker, S.; Nithya, R.S.; Anuja, M.M.; Rejitha, S.; Indira, M. Antiperoxidative and antiinflammatory effect of Sida cordifolia linn. On quinolinic acid induced neurotoxicity. Neurochem. Res. 2010, 35, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S. Prostanoids and inflammation: A new concept arising from receptor knockout mice. J. Mol. Med. 2009, 87, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Svensson, J.; Samuelsson, B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. USA 1975, 72, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhao, G.; Christman, J.W.; Xiao, L.; Van Breemen, R.B. Method development and validation for ultra-high pressure liquid chromatography/tandem mass spectrometry determination of multiple prostanoids in biological samples. J. AOAC Int. 2013, 96, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Zhou, X.; Wong, H.L.; Ng, C.F.; Fu, W.M.; Leung, P.C.; Peng, G.; Ko, C.H. In vivo and in vitro anti-inflammatory effects of zao-jiao-ci (the spine of Gleditsia sinensis lam.) aqueous extract and its mechanisms of action. J. Ethnopharmacol. 2016, 192, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cesar, F.A.; Rudnicki, M.; de Las Heras, B.; Bosca, L.; Lima, M.C.; Pitta, I.R.; Abdalla, D.S. New indole-thiazolidine attenuates atherosclerosis in ldlr(−/−) mice. Vasc. Pharmacol. 2015, 71, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.M.; Martins, D.T.O.; Balogun, S.O.; Oliveira, R.G.; Ascencio, S.D.; Soares, I.M.; Barbosa, R.D.S.; Ademowo, O.G. Ocimum gratissimum l. Leaf flavonoid-rich fraction suppress lps-induced inflammatory response in raw 264.7 macrophages and peritonitis in mice. J. Ethnopharmacol. 2017, 204, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Brazil. Farmacopeia Brasileira; Ateneu: São Paulo, Brazil, 2005. [Google Scholar]
- Brazil. Formulário de Fitoterápicos Farmacopeia Brasileira; Agência Nacional de Vigilância Sanitária—ANVISA: Brasilia, Brazil, 2011.
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Castillo, J.; Lozano, M.L.; Martínez, C.; Benavente-García, O.; Vicente, V.; Rivera, J. Thromboxane a2receptor antagonism by flavonoids: Structure−activity relationships. J. Agric. Food Chem. 2009, 57, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.A.; Navarro-Nuñez, L.; Lozano, M.L.; Martínez, C.; Vicente, V.; Gibbins, J.M.; Rivera, J. Flavonoids inhibit the platelet txa2signalling pathway and antagonize txa2receptors (tp) in platelets and smooth muscle cells. Br. J. Clin. Pharmacol. 2007, 64, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.K.; Wahl, P.; Bilic, G.; Wüthrich, R.P. Cd44-mediated cyclooxygenase-2 expression and thromboxane a2 production in raw 264.7 macrophages. Inflamm. Res. 2001, 50, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (tnf-α) and interleukin-10 (il-10) in raw-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Nunes, X.P.; Azevedo Maia, G.L.; Silva Almeida, J.R.G.; Pereira, F.O.; Lima, E.O. Antimicrobial activity of the essential oil of Sida cordifolia L. Rev. Bras. Farmacogn. 2006, 16, 3. [Google Scholar] [CrossRef]
- Breitbach, U.B.; Niehues, M.; Lopes, N.P.; Faria, J.E.Q.; Brandão, M.G.L. Amazonian brazilian medicinal plants described by c.F.P. Von martius in the 19th century. J. Ethnopharmacol. 2013, 147, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin e2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Almowallad, F.M.; Esmat, A.; Shehata, I.A.; Abdel-Sattar, E.A. Anti-inflammatory activity of flavonoids from chrozophora tinctoria. Phytochem. Lett. 2015, 13, 74–80. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanevello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Bueno, F.G.; Panizzon, G.P.; Mello, E.V.S.d.L.; Lechtenberg, M.; Petereit, F.; Mello, J.C.P.d.; Hensel, A. Hydrolyzable tannins from hydroalcoholic extract from poincianella pluviosa stem bark and its wound-healing properties: Phytochemical investigations and influence on in vitro cell physiology of human keratinocytes and dermal fibroblasts. Fitoterapia 2014, 99, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shu, Z.; Yin, L.; Ma, L.; Wang, X.; Fu, X. Anti-inflammatory and antinociceptive activities of non-alkaloids fractions from aconitum flavum in vivo. Rev. Bras. Farmacogn. 2015, 25, 47–52. [Google Scholar] [CrossRef]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and change in the mediterranean medical tradition: Ruta spp. (rutaceae) in hippocratic medicine and present practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Camejo-Rodrigues, J.; Ascensão, L.; Bonet, M.À.; Vallès, J. An ethnobotanical study of medicinal and aromatic plants in the natural park of “serra de são mamede” (portugal). J. Ethnopharmacol. 2003, 89, 199–209. [Google Scholar] [CrossRef]
- Daswani, P.G.; Ghadge, A.A.; Birdi, T.J. Preparation of decoction of medicinal plants: A self-help measure? J. Altern. Complement. Med. 2011, 17, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wang, H.; Jiang, Z.; Huo, M.; Yan, C.; Zheng, C.; Gu, N. Integrated pharmacokinetics and biodistribution of multiple flavonoid c-glycosides components in rat after oral administration of abrus mollis extract and correlations with bio-effects. J. Ethnopharmacol. 2015, 163, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Madgula, V.; Avula, B.; Pawar, R.; Shukla, Y.; Khan, I.; Walker, L.; Khan, S. In vitrometabolic stability and intestinal transport of p57as3 (p57) fromhoodia gordoniiand its interaction with drug metabolizing enzymes. Planta Med. 2008, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A. Anti-Inflammatory Activity of “Malvas” Species and e Multivariate Analysis for Quality Control of Commercial Samples; Federal University of Paraná—UFPR: Curitiba, Brazil, 2015. [Google Scholar]
- Jeon, Y.J.; Han, S.H.; Lee, Y.W.; Lee, M.; Yang, K.H.; Kim, H.M. Dexamethasone inhibits il-1 beta gene expression in lps-stimulated raw 264.7 cells by blocking nf-kappa b/rel and ap-1 activation. Immunopharmacology 2000, 48, 173–183. [Google Scholar] [CrossRef]
- Lin, I.C.; Kuo, C.-D. Pro-inflammatory effects of commercial alpha-lactalbumin on raw 264.7 macrophages is due to endotoxin contamination. Food Chem. Toxicol. 2010, 48, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Noh, E.J.; Cha, K.-H.; Kim, Y.S.; Lim, S.S.; Shin, K.H.; Jung, S.H. Inhibitory effects of irigenin from the rhizomes of belamcanda chinensis on nitric oxide and prostaglandin e2 production in murine macrophage raw 264.7 cells. Life Sci. 2006, 78, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Yi, L.; Wang, Q.; Xie, B.-B.; Dong, Y.; Sha, C.-W. Anti-inflammatory effects of physalin e from physalis angulata on lipopolysaccharide-stimulated raw 264.7 cells through inhibition of nf-κb pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 74–79. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Species | HF | CF | EAF | RF |
|---|---|---|---|---|
| M. sylvestris | 48.1 | 13.0 | 11.7 | 27.2 |
| S. cordifolia | 47.7 | 7.8 | 7.0 | 37.5 |
| P. graveolens | 40.2 | 11.5 | 13.4 | 34.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, C.A.F.; Campos, M.L.; Irioda, A.C.; Stremel, D.P.; Trindade, A.C.L.B.; Pontarolo, R. Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production. Molecules 2017, 22, 1883. https://doi.org/10.3390/molecules22111883
Martins CAF, Campos ML, Irioda AC, Stremel DP, Trindade ACLB, Pontarolo R. Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production. Molecules. 2017; 22(11):1883. https://doi.org/10.3390/molecules22111883
Chicago/Turabian StyleMartins, Cleverson Antonio Ferreira, Michel Leandro Campos, Ana Carolina Irioda, Dile Pontarolo Stremel, Angela Cristina Leal Badaró Trindade, and Roberto Pontarolo. 2017. "Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production" Molecules 22, no. 11: 1883. https://doi.org/10.3390/molecules22111883