Construction of Luminogen Exhibiting Multicolored Emission Switching through Combination of Twisted Conjugation Core and Donor-Acceptor Units
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthsis of Compound 1
2.2. The UV-Vis Absorption and Aggregation-Induced Emission (AIE)
2.3. Emission of the Three Crystals and the Amorphous Solid
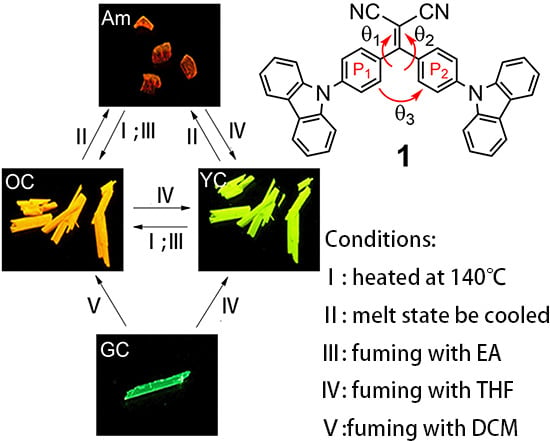
2.4. Tuning the Emission of 1 in the Solid State
2.5. Mechanochromic Fluorescence
2.6. Application in Optical Recording
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Compound 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirata, S.; Watanabe, T. Reversible thermo-responsive recording of fluorescent images (TRF). Adv. Mater. 2006, 18, 2725–2729. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Ren, Z.; Xie, G.; Li, Q.; Li, Z. Synthesis of solution processable blue AIEgens and the device performance. Acta Chim. Sin. 2016, 74, 865–870. [Google Scholar] [CrossRef]
- Huang, J.; Nie, H.; Zeng, J.J.; Zhuang, Z.Y.; Gan, S.F.; Cai, Y.J.; Guo, J.J.; Su, S.J.; Zhao, Z.J.; Tang, B.Z. Highly efficient nondoped OLEDs with negligible efficiency roll-off fabricated from aggregation-induced delayed fluorescence luminogens. Angew. Chem. Int. Ed. 2017, 56, 12971–12976. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.W.; Chen, L.; Peng, H.R.; Chen, S.M.; Zhuang, Z.Y.; Li, Y.H.; Wang, B.H.; Zhao, Z.J.; Tang, B.Z. 3,4-donor- and 2,5-acceptor-functionalized dipolar siloles: Synthesis, structure, photoluminescence and electroluminescence. J. Mater. Chem. C 2017, 5, 4867–4874. [Google Scholar] [CrossRef]
- Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater. 2005, 4, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lei, L.; Zheng, C.; Wei, B.; Zhao, Z.; Qin, A.; Hu, R.; Tang Ben, Z. Tetraphenylethene-containing alkynone derivatives: Design and synthesis, aggregation-induced emission characteristics, and the selective fluorescence detection of Pd2+. Acta Chim. Sin. 2016, 74, 885–892. [Google Scholar] [CrossRef]
- Ji, G.; Yan, L.; Wang, H.; Ma, L.; Xu, B.; Tian, W. Efficient near-infrared AIE nanoparticles for cell imaging. Acta Chim. Sin. 2016, 74, 917–922. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, Y.; Wang, Z.; Tong, B.; Shi, J.; Dong, Y. Properties of polymorphism and acid response of pyrrolopyrrole-based derivative with aggregation-induced emission behavior. Acta Chim. Sin. 2016, 74, 942–948. [Google Scholar] [CrossRef]
- Luo, H.Y.; Chen, J.H.; Liu, C.; Shi, G.; Li, G.M.; Chi, Z.G. Synthesis and properties of a multi-functional luminescent material with carbazolyl and tetraphenylethylene moiety. Acta Polym. Sin. 2017, 1277–1284. [Google Scholar]
- Yang, P.-P.; Dong, L.-C.; Li, Y.-Y.; Zhang, L.-L.; Shi, J.-B.; Zhi, J.-G.; Tong, B.; Dong, Y.-P. Synthesis and aggregation-enhanced emission of polymethacrylate with pentaphenylpyrrole side group. Acta Polym. Sin. 2017, 8, 1285–1293. [Google Scholar]
- Khandare, D.G.; Joshi, H.; Banerjee, M.; Majik, M.S.; Chatterjee, A. An aggregation-induced emission based “turn-on” fluorescent chemodosimeter for the selective detection of Pb2+ ions. RSC Adv. 2014, 4, 47076–47080. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, Y.; Liang, H.; Ho, C.-L.; Zhao, Q.; Huang, W.; Wong, W.-Y. A water-soluble tetraphenylethene based probe for luminescent carbon dioxide detection and its biological application. J. Mater. Chem. C 2015, 3, 11850–11856. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z.-Q.; Gong, W.-L.; Li, C.; Zhu, M.-Q. Ultrasensitive water sensors based on fluorenone-tetraphenylethene AIE luminogens. Mater. Chem. Front. 2017, 1, 1841–1846. [Google Scholar] [CrossRef]
- Gan, S.; Zhou, J.; Smith, T.A.; Su, H.; Luo, W.; Hong, Y.; Zhao, Z.; Tang, B.Z. New AIEgens with delayed fluorescence for fluorescence imaging and fluorescence lifetime imaging of living cells. Mater. Chem. Front. 2017, 1, 2554–2558. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Haeussler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “light-up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem. Commun. 2006, 35, 3705–3707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, H.; Fan, Y.; Zhou, T.; Su, Z.; Liu, Y.; Wang, Y. Thermally induced reversible phase transformations accompanied by emission switching between different colors of two aromatic-amine compounds. Adv. Mater. 2009, 21, 3165–3169. [Google Scholar] [CrossRef]
- Seeboth, A.; Loetzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic polymers-function by design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhu, D.; Zhang, H.; Lu, X.; Lu, Q. Thermally tunable circular dichroism and circularly polarized luminescence of tetraphenylethene with two cholesterol pendants. J. Mater. Chem. C 2015, 3, 6997–7003. [Google Scholar] [CrossRef]
- Takahashi, E.; Takaya, H.; Naota, T. Dynamic vapochromic behaviors of organic crystals based on the open-close motions of s-shaped donor-acceptor folding units. Chem. Eur. J. 2010, 16, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Sun, C.; Harris, K.J.; Lucier, B.E.G.; Schurko, R.W.; Wang, S. Probing the structural origins of vapochromism of a triarylboron-functionalized platinum(ii) acetylide by optical and multinuclear solid-state NMR spectroscopy. Inorg. Chem. 2011, 50, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Minei, P.; Koenig, M.; Battisti, A.; Ahmad, M.; Barone, V.; Torres, T.; Guldi, D.M.; Brancato, G.; Bottari, G.; Pucci, A. Reversible vapochromic response of polymer films doped with a highly emissive molecular rotor. J. Mater. Chem. C 2014, 2, 9224–9232. [Google Scholar] [CrossRef] [Green Version]
- Mizoshita, N.; Tani, T.; Inagaki, S. Isothermally reversible fluorescence switching of a mechanochromic perylene bisimide dye. Adv. Mater. 2012, 24, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-L.; Yao, D.-D.; Zhou, T.-L.; Zhang, H.-Y.; Wang, Y. Reversible piezo- and photochromic behaviors accompanied by emission color switching of two anthracene-containing organic molecules. Chem. Commun. 2011, 47, 7782–7784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chi, Z.; Zhang, J.; Li, H.; Xu, B.; Li, X.; Liu, S.; Zhang, Y.; Xu, J. Piezofluorochromic properties and mechanism of an aggregation-induced emission enhancement compound containing N-hexyl-phenothiazine and anthracene moieties. J. Phys. Chem. B 2011, 115, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, H.Y.; Chi, Z.G.; Zhang, X.Q.; Zhang, J.Y.; Xu, B.J.; Zhang, Y.; Liu, S.W.; Xu, J.R. Piezofluorochromism and morphology of a new aggregation-induced emission compound derived from tetraphenylethylene and carbazole. New J. Chem. 2012, 36, 685–693. [Google Scholar] [CrossRef]
- Duan, Y.; Xiang, X.; Dong, Y. Diphenyldibenzofulvene derivatives exhibiting reversible multicolored mechanochromic luminescence with high contrast. Acta Chim. Sin. 2016, 74, 923–928. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Chen, H.Y.; Qiu, C.F.; Kwok, H.S.; Zhan, X.W.; Liu, Y.Q.; Zhu, D.B.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.W.; Yu, G.; Lee, P.P.S.; Liu, Y.Q.; Zhu, D.B. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lam, J.W.Y.; Qin, A.; Li, Z.; Sun, J.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: Crystallization-induced emission enhancement. Chem. Commun. 2007, 1, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Lam, J.W.Y.; Li, Z.; Qin, A.J.; Tong, H.; Dong, Y.P.; Feng, X.D.; Tang, B.Z. Vapochromism of hexaphenylsilole. J. Inorg. Organomet. Polym. Mater. 2005, 15, 287–291. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y.-N.; Dong, Y.Q.; He, C.; Wang, H. Surfactant-assisted self-assembled polymorphs of AIEgen di(4-propoxyphenyl)dibenzofulvene. J. Mater. Chem. C 2017, 5, 557–565. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Song, G.; He, C.; Dong, Y.Q.; Wang, H. Freezing-induced multi-colour emissions of AIE luminogen di(4-propoxyphenyl) dibenzofulvene. J. Mater. Chem. C 2015, 3, 2677–2685. [Google Scholar] [CrossRef]
- Morris, W.A.; Butler, T.; Kolpaczynska, M.; Fraser, C.L. Stimuli responsive furan and thiophene substituted difluoroboron beta-diketonate materials. Mater. Chem. Front. 2017, 1, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Huang, K.; Tao, Y.; Li, X.; Zhang, L.; Lu, P.; Wang, Y. Turning on the solid emission from non-emissive 2-aryl-3-cyanobenzofurans by tethering tetraphenylethene for green electroluminescence. Mater. Chem. Front. 2017, 1, 1858–1865. [Google Scholar] [CrossRef]
- Wang, F.; DeRosa, C.A.; Daly, M.L.; Song, D.; Sabat, M.; Fraser, C.L. Multi-stimuli responsive luminescent azepane-substituted β-diketones and difluoroboron complexes. Mater. Chem. Front. 2017, 1, 1866–1874. [Google Scholar] [CrossRef]
- Toal, S.J.; Jones, K.A.; Magde, D.; Trogler, W.C. Luminescent silole nanoparticles as chemoselective sensors for Cr (VI). J. Am. Chem. Soc. 2005, 127, 11661–11665. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.; Fang, X.; Zhang, Y.; Chen, P.; Wang, Y.; Yang, B.; Xu, B.; Tian, W.; Zhang, S.X.-A. AIE (AIEE) and mechanofluorochromic performances of TPE-methoxylates: Effects of single molecular conformations. RSC Adv. 2013, 3, 7996–8002. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, T.; Jiang, S.; Liu, Z.; Fang, D.; Dong, Y.Q. The construction of a multicolored mechanochromic luminogen with high contrast through the combination of a large conjugation core and peripheral phenyl rings. J. Mater. Chem. C 2016, 4, 4800–4804. [Google Scholar] [CrossRef]
- Tian, H.; Wang, P.; Liu, J.; Duan, Y.; Dong, Y.Q. Construction of a tetraphenylethene derivative exhibiting high contrast and multicolored emission switching. J. Mater. Chem. C 2017, in press. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Li, C.; Liu, Z.; Bo, Z.; Dong, Y.; Dong, Y.; Tang, B.Z. Switching emissions of two tetraphenylethene derivatives with solvent vapor, mechanical, and thermal stimuli. Chin. Sci. Bull. 2013, 58, 2723–2727. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Li, B.; Ye, L.; Xu, B.; Zou, B.; Tian, W. Multi-stimuli responsive fluorescence switching: The reversible piezochromism and protonation effect of a divinylanthracene derivative. J. Mater. Chem. C 2013, 1, 7554–7559. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Hu, R.; Sun, J.Z.; Qin, A.; Tang, B.Z. Click synthesis, aggregation-induced emission, E/Z isomerization, self-organization, and multiple chromisms of pure stereoisomers of a tetraphenylethene-cored luminogen. J. Am. Chem. Soc. 2012, 134, 9956–9966. [Google Scholar] [CrossRef] [PubMed]
- Bures, F.; Schweizer, W.B.; May, J.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Biaggio, I.; Diederich, F. Property tuning in charge-transfer chromophores by systematic modulation of the spacer between donor and acceptor. Chem. Eur. J. 2007, 13, 5378–5387. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Villafiorita-Monteleone, F.; Pacini, A.; Botta, C.; Virgili, T.; Forni, A.; Cariati, E.; Boiocchi, M.; Pasini, D. Structure-activity relationship for the solid state emission of a new family of “push-pull” π-extended chromophores. Faraday Discuss. 2017, 196, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Benedini, S.; Carlucci, L.; Forni, A.; Marinotto, D.; Nitti, A.; Pasini, D.; Righetto, S.; Cariati, E. Polymorphism-dependent aggregation induced emission of a push-pull dye and its multi-stimuli responsive behavior. J. Mater. Chem. C 2016, 4, 2979–2989. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; et al. Piezochromic luminescence based on the molecular aggregation of 9,10-bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew. Chem. Int. Ed. 2012, 51, 10782–10785. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.-G.; Kim, D.; Park, S.Y. Multistimuli two-color luminescence switching via different slip-stacking of highly fluorescent molecular sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Tan, Y.; Gong, Y.; Lu, P.; Lam, J.W.Y.; Shen, X.Y.; Feng, C.; Sung, H.H.Y.; Lu, Y.; Williams, I.D.; et al. Synergy between twisted conformation and effective intermolecular interactions: Strategy for efficient mechanochromic luminogens with high contrast. Adv. Mater. 2013, 25, 2837–2843. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, L.; Nie, H.; Tong, J.; Yan, L.; Xu, B.; Sun, J.Z.; Tian, W.; Zhao, Z.; Qin, A.; et al. Tetraphenylpyrazine-based AIEgens: Facile preparation and tunable light emission. Chem. Sci. 2015, 6, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Zhang, W.; Wang, X.; Wu, Y.; Liang, T.; Hao, X.; Fu, H.; Zhao, Y.; Zhang, D. Tuning the solid state emission of the carbazole and cyano-substituted tetraphenylethylene by co-crystallization with solvents. Small 2016, 12, 6554–6561. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound (2-(bis(4-(carbazol-9-yl)phenyl)methylene)malononitrile (1) are available from the authors. |







| Samples | a λem (nm) | b ΦF (%) | c ⟨τ⟩ (ns) |
|---|---|---|---|
| 1GC | 506 | 19.8 | 6.59 |
| 1YC | 537 | 17.8 | 18.66 |
| 1OC | 585 | 30.0 | 23.23 |
| 1Am | 585 | 13.9 | 17.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Tang, X.; Dong, Y.Q. Construction of Luminogen Exhibiting Multicolored Emission Switching through Combination of Twisted Conjugation Core and Donor-Acceptor Units. Molecules 2017, 22, 2222. https://doi.org/10.3390/molecules22122222
Tian H, Tang X, Dong YQ. Construction of Luminogen Exhibiting Multicolored Emission Switching through Combination of Twisted Conjugation Core and Donor-Acceptor Units. Molecules. 2017; 22(12):2222. https://doi.org/10.3390/molecules22122222
Chicago/Turabian StyleTian, Haiyan, Xi Tang, and Yong Qiang Dong. 2017. "Construction of Luminogen Exhibiting Multicolored Emission Switching through Combination of Twisted Conjugation Core and Donor-Acceptor Units" Molecules 22, no. 12: 2222. https://doi.org/10.3390/molecules22122222