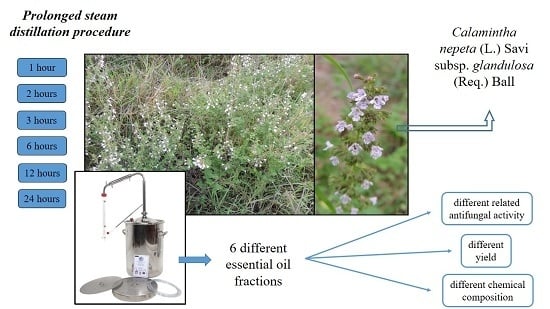
Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches
Abstract
:1. Introduction
Taxonomic Characterization and Uses of C. nepeta (L.) Savi and Its Subspecies
2. Results and Discussion
2.1. EO Extraction
2.2. GC/MS Analysis
2.3. Anti-Candida Activity
3. Materials and Methods
3.1. Plant Material
3.2. EO Extraction
3.3. EO Analysis
3.4. Antimicrobial Assay
3.5. Statistical Evaluations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Mahmood, M.S.; Akhtar, M. In vitro and in vivo evaluation of antimicrobial activities of essential oils extracted from some indigenous spices. Pak. Vet. J. 2013, 33, 413–417. [Google Scholar]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary approach to determine the optimal time and period for extracting the essential oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef] [PubMed]
- Ball, P.W.; Getliffe, F. Calamintha Miller. In Flora Europaea; Tutin, T., Heywood, V., Burges, N., Moore, D., Valentine, D., Walters, S., Webb, D., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 3, pp. 166–167. [Google Scholar]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova, J. Essential Oils of Calamintha nepeta subsp. nepeta and subsp. glandulosa from Corsica (France). J. Essent. Oil Res. 1996, 8, 363–366. [Google Scholar] [CrossRef]
- Şarer, E.; Solakel Pançalı, S. Composition of the essential oil from Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) P. W. Ball. Flavour Fragr. J. 1998, 13, 31–32. [Google Scholar]
- Alan, S.; Kürkҫüoglu, M.; Hüsnü, K.; Başer, K. Composition of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta and Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball. Asian J. Chem. 2011, 23, 2357–2360. [Google Scholar]
- Demirci, B.; Temel, H.; Portakal, T.; Kırmızıbekmez, H.; Demirci, F.; Başer, K. Inhibitory effect of Calamintha nepeta subsp. glandulosa essential oil on lipoxygenase. Turk. J. Biochem. 2011, 36, 290–295. [Google Scholar]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological assays of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Notarnicola, S.; De Bellis, L.; Miceli, A. Intraspecific variability of the essential oil of Calamintha nepeta subsp. nepeta from Southern Italy (Apulia). Nat. Prod. Res. 2013, 27, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Souleles, C.; Argyriadou, N.; Philianos, S. Constituents of the essential oil of Calamintha nepeta. J. Nat. Prod. 1987, 50, 510–511. [Google Scholar] [CrossRef]
- De Pooter, H.; Goetghebeur, P.; Schamp, N. Variability in composition of the essential oil of Calamintha nepeta. Phytochemistry 1987, 26, 3355–3356. [Google Scholar] [CrossRef]
- Ćavar, S.; Vidić, D.; Maksimović, M. Volatile constituents, phenolic compounds, and antioxidant activity of Calamintha glandulosa (Req.) Bentham. J. Sci. Food Agric. 2012, 93, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F.; Uzunov, D.; Solimene, U.; Menichini, F. Comparative chemical composition and antioxidant activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Nyman and Calamintha grandiflora (L.) Moench (Labiatae). Nat. Prod. Res. 2012, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.; Puleio, R.; Morelli, I.; Panizzi, L. Antimicrobial activity of the essential oil of Calamintha nepeta and its constituent pulegone against bacteria and fungi. Phytother. Res. 1999, 13, 349–351. [Google Scholar] [CrossRef]
- Kitić, D.; Jovanović, T.; Ristić, M.; Palić, R.; Stojanović, G. Chemical composition and antimicrobial activity of the essential oil of Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) P.W. Ball from Montenegro. J. Essent. Oil Res. 2002, 14, 150–152. [Google Scholar]
- Sarac, N.; Ugur, A. The In Vitro antimicrobial activities of the essential oils of some Lamiaceae species from Turkey. J. Med. Food 2009, 12, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Ceker, S.; Agar, G.; Alpsoy, L.; Nardemir, G.; Kizil, H. Protective role of essential oils of Calamintha nepeta L. on oxidative and genotoxic demage caused by Alfatoxin B1 in vitro. Fresenius Environ. Bull. 2013, 22, 3258–3263. [Google Scholar]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal herbs for the treatment of rheumatic disorders—A survey of European herbals from the 16th and 17th century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.; Morelli, I. Composition and antimicrobial properties of essential oils of four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef]
- Stanić, G.; Blažević, N.; Brkić, D.; Lukač, G. The composition of essential oils of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P. W. Ball and Calamintha sylvatica Bromf. subsp. sylvatica. Acta. Pharm. 1999, 49, 107–112. [Google Scholar]
- Akgül, A.; De Pooter, H.; De Buyck, L. The Essential oils of Calamintha nepeta subsp. glandulosa and Ziziphora clinopodioides from Turkey. J. Essent. Oil Res. 1991, 3, 7–10. [Google Scholar]
- Couladis, M.; Tzakou, O. Essential oil of Calamintha nepeta subsp. glandulosa from Greece. J. Essent. Oil Res. 2001, 13, 11–12. [Google Scholar] [CrossRef]
- Kokkalou, E.; Stefanou, E. The volatile oil of Calamintha nepeta (L.) savi subsp. glandulosa (req.) P. W. Ball, endemic to Greece. Flavour Fragr. J. 1990, 5, 23–26. [Google Scholar] [CrossRef]
- Kirimer, N.; Baser, K.; Özek, T.; Kürkçüoglu, M. Composition of the essential oil of Calamintha nepeta subsp. glandulosa. J. Essent. Oil Res. 1992, 4, 189–190. [Google Scholar] [CrossRef]
- De Pooter, H.; De Buyck, L.; Schamp, N. The volatiles of Calamintha nepeta subsp. glandulosa. Phytochemistry 1986, 25, 691–694. [Google Scholar] [CrossRef]
- Riela, S.; Bruno, M.; Formisano, C.; Rigano, D.; Rosselli, S.; Saladino, M.; Senatore, F. Effects of solvent-free microwave extraction on the chemical composition of essential oil of Calamintha nepeta (L.) Savi compared with the conventional production method. J. Sep. Sci. 2008, 31, 1110–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagni, A.; Catalano, S.; Cioni, P.; Coppi, C.; Morelli, I. Etudes morpho-anatomiques et phytochimiques de Calamintha nepeta (L.) Savi (Labiétes). Plant. Med. Phytothér. 1990, 24, 203–213. [Google Scholar]
- Baser, K.; Kirimer, N.; Tümen, G. Pulegone-rich essential oils of Turkey. J. Essent. Oil Res. 1998, 10, 1–8. [Google Scholar] [CrossRef]
- José Pérez-Alonso, M.; Velasco-Negueruela, A.; López Sáez, J. The volatiles of two Calamintha species growing in Spain, C. sylvatica Bromf. and C. nepeta (L.) Savi. Acta Hortic. 1993, 333, 255–260. [Google Scholar] [CrossRef]
- Asfaw, N.; Storesund, H.; Skattebøl, L.; Aasen, A. Coexistence of chrysanthenone, filifolone and (Z)-isogeranic acid in hydrodistillates. Artefacts! Phytochemistry 2001, 58, 489–492. [Google Scholar] [CrossRef]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Sahin, F.; Agar, G. Chemical composition and antibacterial activity of essential oils of two species of Lamiaceae against phytopathogenic bacteria. Pol. J. Microbio. 2015, 64, 121–127. [Google Scholar]
- Foto, E.; Zilifdar, F.; Yeșilyurt, E.; Biypi, B.; Diril, N. The in vitro antibacterial activity of some extracts and fractions of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball (Lamiaceae). In Proceedings of the 11th International Symposium of Pharmaceutical Sciences, Ankara, Turkey, 9–12 June 2015; Ankara University Faculty of Pharmacy Publication, Book of Abstracts. ; pp. 397–398.
- Neto, A.; Netto, J.; Pereira, P.; Pereira, A.; Taleb-Contini, S.; França, S.; Marques, M.O.; Beleboni, R.O. The role of polar phytocomplexes on anticonvulsant effects of leaf extracts of Lippia alba (Mill.) N.E. Brown chemotypes. J. Pharm. Pharmacol. 2009, 61, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. A multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flavour Fragr. J. 2013, 28, 144–154. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.; Haroutounian, S. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.; Haroutounian, S. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, S. Flora d'Italia; Edagricole: Bologna, Italy, 1982; pp. 1–732. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.

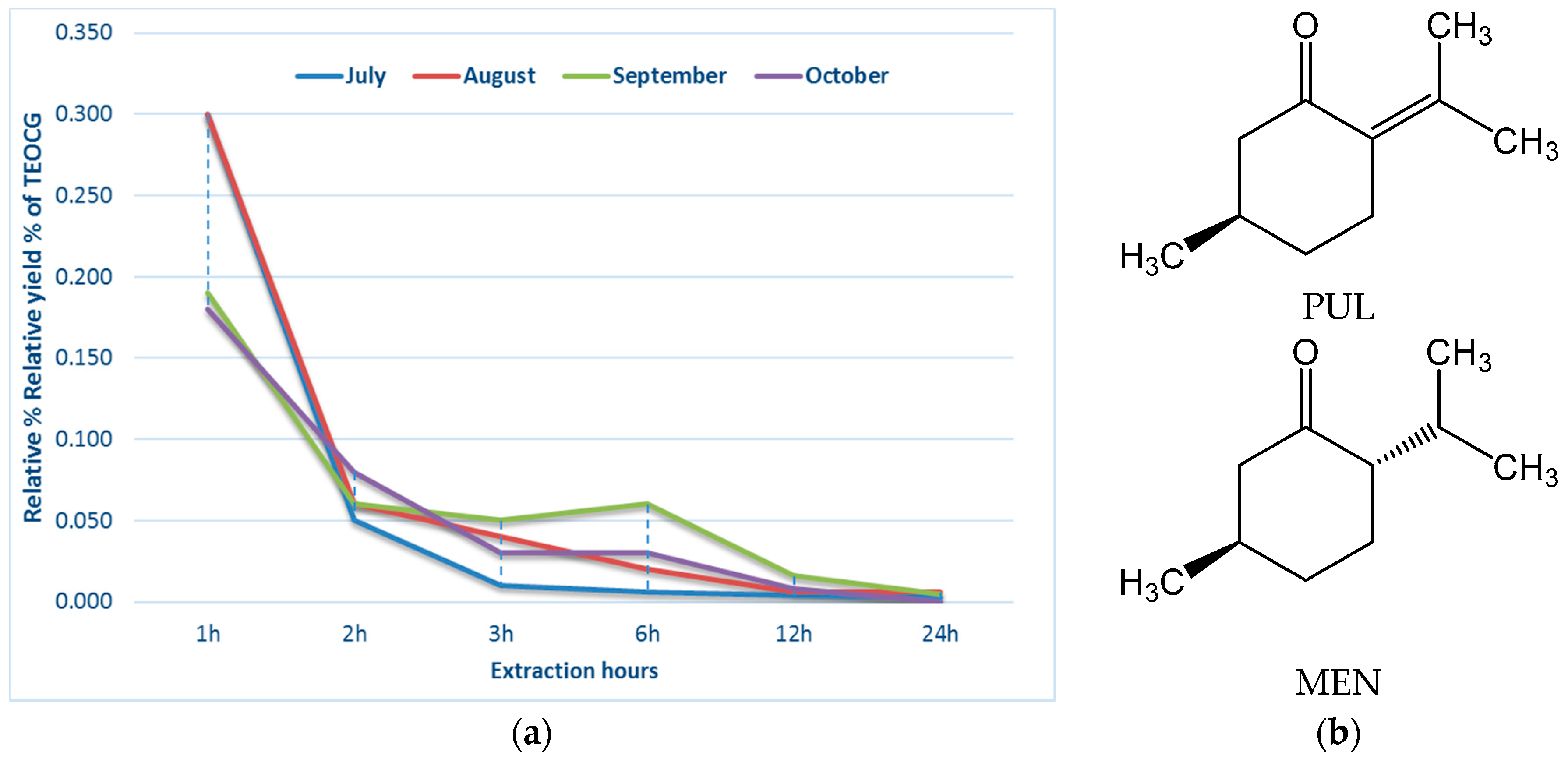
| h 1 | July 2 | August | September | October |
|---|---|---|---|---|
| 1 | 0.300 | 0.300 | 0.190 | 0.180 |
| 2 | 0.350 | 0.360 | 0.250 | 0.260 |
| 3 | 0.360 | 0.400 | 0.300 | 0.290 |
| 6 | 0.366 | 0.420 | 0.360 | 0.320 |
| 12 | 0.370 | 0.426 | 0.376 | 0.328 |
| 24 | 0.373 | 0.432 | 0.381 | 0.328 |
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| J1h | J2h | J3h | J6h | J12h | J24h | ||
| 4 | 3-octanol | 2.2 ± 0.14 | 0.4 ± 0.03 | 0.3 ± 0.02 | 0.4 ± 0.03 | 0.5 ± 0.03 | - |
| 5 | terpinen-4-ol | 0.6 ± 0.04 | 0.5 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.02 | - |
| 15 | cinerolone | - | - | - | 2.9 ± 0.21 | 5.8 ± 0.42 | - |
| 17 | crysanthenone | 4.4 ± 0.41 | 10.5 ± 0.98 | 20.3 ± 1.89 | 22.7 ± 2.11 | 33.9 ± 3.15 | 27.3 ± 2.54 |
| 18 | δ-cadinene | - | - | - | 0.6 ± 0.04 | 0.8 ± 0.05 | 2.4 ± 0.16 |
| 19 | limonene | 5.9 ± 0.57 | 0.6 ± 0.06 | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 | - |
| 20 | germacrene D | - | - | 1.5 ± 0.08 | 2.7 ± 0.14 | 0.8 ±0.04 | - |
| 21 | isocaryophyllene | 0.3 ± 0.02 | - | 1.3 ± 0.11 | 2.9 ± 0.24 | 2.3 ± 0.19 | 3.8 ± 0.36 |
| 22 | isopiperitenone | - | - | - | - | - | 2.2 ± 0.19 |
| 23 | isopulegone | 0.6 ± 0.05 | 0.5 ± 0.05 | 0.5 ± 0.05 | 0.5 ± 0.05 | 0.4 ± 0.04 | - |
| 24 | linalool | 0.3 ± 0.02 | - | 0.2 ± 0.01 | 0.4 ± 0.02 | 0.5 ± 0.03 | - |
| 26 | menthone | 3.1 ± 0.25 | 0.8 ± 0.06 | 0.6 ± 0.05 | 0.6 ± 0.05 | 0.5 ± 0.04 | - |
| 27 | methylisopulegone | - | - | - | - | - | 12.6 ± 0.76 |
| 28 | myrcene | 0.4 ± 0.03 | - | - | - | - | - |
| 29 | p-cymen-8-ol | - | - | - | 0.7 ± 0.06 | 1.5 ± 0.13 | 2.2 ± 0.19 |
| 33 | p-mentha-1,8-dien-3-one | - | 0.6 ± 0.03 | 0.7 ± 0.04 | 1.2 ± 0.07 | 2.0 ± 0.12 | - |
| 34 | p-menthene | - | - | - | 0.2 ± 0.02 | - | - |
| 36 | pulegone | 76.8 ± 5.91 | 77.7 ± 5.98 | 64.3 ± 4.95 | 53.2 ± 4.09 | 41.1 ± 3.16 | 37.7 ± 2.90 |
| 37 | sabinene | 0.6 ± 0.05 | - | - | - | - | - |
| 38 | α-terpineol | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.7 ± 0.04 | 0.8 ± 0.05 | 1.2 ± 0.07 | - |
| 39 | trans-p-mentha-2,8-dienol | - | - | - | 0.2 ± 0.02 | 0.1 ± 0.01 | - |
| Unidentified compounds | 4.5 ± 0.25 | 7.9 ± 0.44 | 9.0 ± 0.50 | 9.5 ± 0.53 | 8.1 ± 0.45 | 11.8 ± 0.66 | |
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| A1h | A2h | A3h | A6h | A12h | A24h | ||
| 4 | 3-octanol | 1.6 ± 0.15 | 0.4 ± 0.04 | 0.2 ± 0.02 | 0.3 ± 0.03 | 0.5 ± 0.05 | 0.5 ± 0.05 |
| 5 | terpinen-4-ol | 0.4 ± 0.04 | 0.4 ± 0.04 | 0.5 ± 0.05 | 0.5 ± 0.05 | 0.7 ± 0.06 | 0.6 ± 0.05 |
| 7 | p-cymene | 0.5 ± 0.05 | 0.4 ± 0.03 | 0.3 ± 0.03 | 0.5 ± 0.05 | 0.3 ± 0.03 | - |
| 9 | β-myrcene | 0.5 ± 0.03 | - | - | - | - | - |
| 12 | β-terpinene | 0.8 ± 0.05 | 0.1 ± 0.01 | - | - | - | - |
| 14 | caryophyllene oxide | - | - | - | 1.2 ± 0.09 | 1.8 ± 0.13 | - |
| 17 | crysanthenone | 2.6 ± 0.25 | 5.2 ± 0.49 | 9.0 ± 0.86 | 18.4 ± 1.77 | 24.0 ± 2.30 | 29.5 ± 2.83 |
| 18 | δ-cadinene | - | - | - | - | 0.5 ± 0.04 | 1.1 ± 0.08 |
| 19 | limonene | 7.5 ± 0.46 | 1.0 ± 0.06 | 0.6 ± 0.04 | 0.6 ± 0.04 | 0.2 ± 0.01 | - |
| 21 | isocaryophyllene | - | - | - | - | 1.3 ± 0.09 | 2.4 ± 0.17 |
| 23 | isopulegone | 0.6 ± 0.05 | 0.6 ± 0.05 | 0.6 ± 0.05 | 0.6 ± 0.05 | 0.8 ± 0.06 | 0.7 ± 0.05 |
| 24 | linalool | 0.4 ± 0.03 | 0.2 ± 0.01 | - | 0.2 ± 0.01 | 0.4 ± 0.03 | 0.3 ± 0.02 |
| 25 | menthol | 0.4 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 |
| 26 | menthone | 3.9 ± 0.37 | 2.1 ± 0.19 | 1.0 ± 0.09 | 0.8 ± 0.08 | 0.7 ± 0.07 | 0.7 ± 0.07 |
| 30 | piperitenone | - | - | - | 0.5 ± 0.03 | 0.8 ± 0.04 | 0.9 ± 0.05 |
| 33 | p-menth-1,8-dien-3-one | - | - | 0.4 ± 0.03 | 0.6 ± 0.04 | 1.0 ± 0.06 | 1.9 ± 0.12 |
| 32 | p-menth-1-en-8-ol | - | 0.5 ± 0.03 | 0.6 ± 0.04 | 1.0 ± 0.07 | 1.6 ± 0.11 | 2.7 ± 0.19 |
| 36 | pulegone | 80.8 ± 5.49 | 84.7 ± 5.76 | 80.0 ± 5.44 | 66.0 ± 4.49 | 55.4 ± 3.77 | 49.9 ± 3.39 |
| Unidentified compounds | 0.0 | 3.9 ± 0.22 | 6.3 ± 0.36 | 8.3 ± 0.47 | 9.6 ± 0.55 | 8.4 ± 0.48 | |
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| S1h | S2h | S3h | S6h | S12h | S24h | ||
| 2 | 2-hydroxypiperitenone | - | - | - | - | - | 1.1 ± 0.09 |
| 4 | 3-octanol | 3.0 ± 0.21 | 1.7 ± 0.12 | 0.8 ± 0.05 | - | - | - |
| 5 | terpinen-4-ol | 0.7 ± 0.06 | 0.6 ± 0.05 | 0.7 ± 0.06 | 0.6 ± 0.05 | 0.9 ± 0.07 | 0.7 ± 0.06 |
| 7 | p-cymene | 0.8 ± 0.08 | - | - | - | - | - |
| 9 | β-myrcene | 0.6 ± 0.06 | - | - | - | - | - |
| 11 | β-pinene | 1.2 ± 0.08 | - | - | - | - | - |
| 14 | caryophyllene oxide | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.6 ± 0.03 | 0.7 ± 0.04 | 1.6 ± 0.08 | 1.8 ± 0.09 |
| 15 | cinerolone | - | - | - | - | - | 2.9 ± 0.5 |
| 17 | crysanthenone | 1.3 ± 0.08 | 2.0 ± 0.13 | 3.4 ± 0.21 | 6.8 ± 0.43 | 13.6 ± 0.86 | 18.6 ± 1.17 |
| 18 | δ-cadinene | - | - | - | - | - | 1.1 ±0.08 |
| 19 | limonene | 13.6 ± 1.33 | 2.1 ± 0.20 | 1.2 ± 0.12 | 0.6 ± 0.06 | 0.7 ± 0.07 | 0.7 ± 0.07 |
| 23 | isopulegone | 1.1 ± 0.07 | 1.5 ± 0.09 | 1.3 ± 0.08 | 1.1 ± 0.07 | 1.1 ± 0.07 | 9.4 ± 0.58 |
| 25 | menthol | 1.3 ± 0.12 | 2.2 ± 0.20 | 1.9 ± 0.18 | 1.9 ± 0.18 | 1.7 ± 0.16 | 1.7 ± 0.16 |
| 26 | menthone | 20.3 ± 1.52 | 20.0 ± 1.50 | 11.2 ± 0.84 | 5.9 ± 0.44 | 4.1 ± 0.31 | 3.7 ± 0.28 |
| 29 | p-cymen-8-ol | - | - | - | - | 0.7 ± 0.06 | 1.5 ± 0.14 |
| 30 | piperitenone | 0.7 ± 0.06 | 1.5 ± 0.13 | 2.0 ± 0.17 | 3.1 ± 0.26 | 4.5 ± 0.38 | 3.9 ± 0.33 |
| 31 | piperitenone oxide | 1.3 ± 0.09 | 1.3 ± 0.09 | 0.8 ± 0.05 | 0.5 ± 0.03 | - | - |
| 33 | p-menth-1,8-dien-3-one | - | - | - | - | 0.9 ± 0.07 | 1.3 ± 0.09 |
| 32 | p-menth-1-en-8-ol | 0.2 ± 0.02 | 0.4 ± 0.03 | 0.5 ± 0.04 | 0.6 ± 0.05 | 1.1 ± 0.09 | 1.6 ± 0.14 |
| 36 | pulegone | 48.8 ± 4.39 | 62.5 ± 5.62 | 72.9 ± 6.56 | 74.9 ± 6.74 | 64.8 ± 5.83 | 43.2 ± 3.89 |
| 37 | sabinene | 1.4 ± 0.14 | - | - | - | - | - |
| Unidentified compounds | 3.4 ± 0.20 | 3.7 ± 0.22 | 2.7 ± 0.16 | 3.3 ± 0.19 | 4.3 ± 0.25 | 6.8 ± 0.40 | |
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| O1h | O2h | O3h | O6h | O12h | O24h | ||
| 3 | 3-metilcicloesanone | - | - | - | - | 0.9 ± 0.06 | 1.9 ± 0.13 |
| 4 | 3-octanol | 2.1 ± 0.19 | 1.0 ± 0.09 | 0.6 ± 0.05 | 0.3 ± 0.03 | 0.3 ± 0.03 | 0.7 ± 0.06 |
| 5 | terpinen-4-ol | 0.6 ± 0.03 | 0.7 ± 0.03 | 0.9 ± 0.04 | 0.9 ± 0.04 | 1.1 ± 0.05 | 0.8 ± 0.04 |
| 14 | caryophyllene oxide | - | - | 1.0 ± 0.06 | 1.2 ± 0.07 | 1.8 ± 0.11 | 1.7 ± 0.11 |
| 17 | crysanthenone | 1.3 ± 0.11 | 2.3 ± 0.19 | 3.3 ± 0.28 | 5.3 ± 0.44 | 5.4 ± 0.45 | 13.4 ± 1.12 |
| 19 | limonene | 9.2 ± 0.51 | 2.1 ± 0.11 | 0.6 ± 0.03 | 0.7 ± 0.04 | 0.8 ± 0.04 | 0.9 ± 0.05 |
| 21 | iso-caryophyllene | 0.4 ± 0.02 | 0.6 ± 0.04 | 0.5 ± 0.03 | 0.7 ± 0.04 | 2.0 ± 0.12 | 3.5 ± 0.21 |
| 23 | isopulegone | 1.0 ± 0.05 | 1.0 ± 0.05 | 1.2 ± 0.06 | 1.1 ± 0.06 | 1.3 ± 0.07 | 1.0 ± 0.05 |
| 25 | menthol | 4.3 ± 0.25 | 4.4 ± 0.25 | 6.3 ± 0.36 | 5.5 ± 0.32 | 5.3 ± 0.31 | 4.2 ± 0.24 |
| 26 | menthone | 35.4 ± 3.01 | 27.8 ± 2.36 | 23.6 ± 2.01 | 10.9 ± 0.93 | 7.0 ± 0.59 | 6.8 ± 0.58 |
| 31 | piperitenone oxide | 1.4 ± 0.14 | 1.9 ± 0.18 | 3.3 ± 0.32 | 3.6 ± 0.35 | 0.5 ± 0.05 | 5.2 ± 0.51 |
| 32 | p-menth-1-en-8-ol | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.8 ± 0.05 | 1.4 ± 0.09 | 2.3 ± 0.15 |
| 36 | pulegone | 42.5 ± 2.17 | 57.5 ± 2.93 | 53.3 ± 2.72 | 68.2 ± 3.48 | 68.8 ± 3.51 | 51.8 ± 2.64 |
| Unidentified compounds | 1.5 ± 0.13 | 0.4 ± 0.03 | 4.9 ± 0.43 | 0.8 ± 0.07 | 3.4 ± 0.29 | 5.8 ± 0.51 | |
| Sample | MIC mg∙mL−1 | PUL % | Sample | MIC mg∙mL−1 | PUL % | ||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||||
| J1h | 6.24 | 6.24 | 76.8 | S1h | 6.24 | 12.48 | 48.8 |
| J2h | 6.24 | 12.48 | 77.7 | S2h | 6.24 | 12.48 | 62.5 |
| J3h | 0.78 | 6.24 | 64.3 | S3h | 3.12 | 12.48 | 72.9 |
| J6h | na | na | 53.2 | S6h | 1.56 | 6.24 | 74.9 |
| J12h | 12.48 | 12.48 | 41.1 | S12h | 3.12 | 12.48 | 64.8 |
| J24h | na | na | 37.7 | S24h | 12.48 | na | 43.2 |
| JM1 | 12.48 | 12.48 | 72.8 | SM1 | 3.12 | 12.48 | 52.4 |
| JM2 | 12.48 | na | 78.6 | SM2 | 3.12 | 3.12 | 55.6 |
| JM3 | 1.56 | 6.24 | 73.4 | SM3 | 1.56 | 6.24 | 60.8 |
| JM4 | 6.24 | 6.24 | 77.6 | SM4 | 0.78 | 6.24 | 53.6 |
| JM5 | 12.48 | 12.48 | 76.2 | SM5 | 3.12 | 6.24 | 55.6 |
| A1h | 3.12 | 12.48 | 80.8 | O1h | 6.24 | 12.48 | 42.5 |
| A2h | 3.12 | 6.24 | 84.7 | O2h | 6.24 | 12.48 | 57.5 |
| A3h | 1.56 | 3.12 | 80.0 | O3h | 6.24 | 12.48 | 53.3 |
| A6h | 3.12 | 6.24 | 66.0 | O6h | 12.48 | 12.48 | 68.2 |
| A12h | 6.24 | na | 55.4 | O12h | 12.48 | 12.48 | 68.8 |
| A24h | 6.24 | 12.48 | 49.9 | O24h | na | na | 51.8 |
| AM1 | 3.12 | 6.24 | 75.7 | OM1 | 6.24 | 12.48 | 10.1 |
| AM2 | 0.78 | 3.12 | 78.8 | OM2 | 6.24 | 12.48 | 50.5 |
| AM3 | 3.12 | 6.24 | 76.9 | OM3 | 12.48 | 12.48 | 52.0 |
| AM4 | 3.12 | 3.12 | 76.4 | OM4 | 12.48 | 12.48 | 49.8 |
| AM5 | 12.48 | 12.48 | 79.4 | OM5 | 6.24 | 12.48 | 52.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches. Molecules 2017, 22, 203. https://doi.org/10.3390/molecules22020203
Božović M, Garzoli S, Sabatino M, Pepi F, Baldisserotto A, Andreotti E, Romagnoli C, Mai A, Manfredini S, Ragno R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches. Molecules. 2017; 22(2):203. https://doi.org/10.3390/molecules22020203
Chicago/Turabian StyleBožović, Mijat, Stefania Garzoli, Manuela Sabatino, Federico Pepi, Anna Baldisserotto, Elisa Andreotti, Carlo Romagnoli, Antonello Mai, Stefano Manfredini, and Rino Ragno. 2017. "Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches" Molecules 22, no. 2: 203. https://doi.org/10.3390/molecules22020203
APA StyleBožović, M., Garzoli, S., Sabatino, M., Pepi, F., Baldisserotto, A., Andreotti, E., Romagnoli, C., Mai, A., Manfredini, S., & Ragno, R. (2017). Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches. Molecules, 22(2), 203. https://doi.org/10.3390/molecules22020203